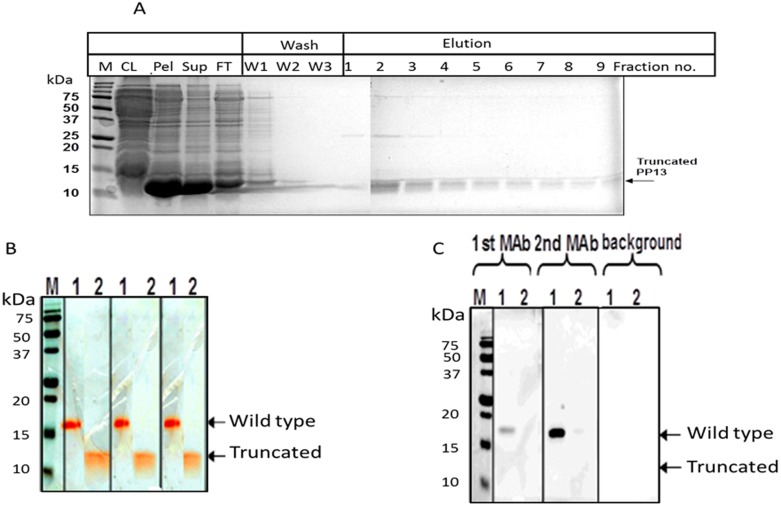
Figure 2. Purification and detection of the wild type and the truncated PP13.
A - Purification and characterization of heterologously expressed His-tagged truncated PP13. Expression of 6 His-tagged PP13 in E. coli was induced by IPTG. The protein was purified on Ni–NTA–agarose under denaturing conditions and separated on 15% SDS–PAGE. The total proteins were visualized by Gel Code blue staining. CL-clear lysate, Pel-cell pellet, Sup-Supernatant, FT-Flow through, W1 - 6 M urea, W2–4 M urea, W3 - without urea. B - The combined eluted fractions were separated by SDS-PAGE and stained by Gel-Code blue and the wildtype and truncated protein are shown. C - Proteins were electrotransferred by Western-blot. Only the His-tagged wild type (lane-1) but not the truncated PP13 (lane-2) was detected with the ELISA capture mAb (mAb1) and the detecting mAb (mAb2) as revealed with marking by HRP-conjugated rabbit anti mouse IgG and ECL detection reagent.