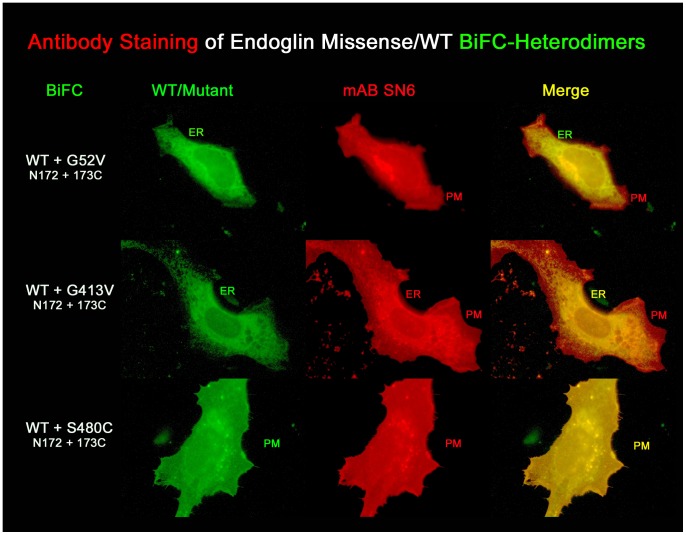
Figure 5. Detection of G52V homodimers as well as G52V/wild-type heterodimers failed with the monoclonal antibody SN6.
In order to selectively visualize mutant/wt heterodimers, CHO cells were co-transfected with endoglin EYFP-BiFC constructs, as indicated. Cells were permeabilized and stained with the endoglin-specific monoclonal antibody (mAb) SN6 (red fluorescence) and analysed for colocalization by fluorescence microscopy. In this experiment, endoglin wild-type homodimers, as well as mutant homodimers are formed, but are not visible by green fluorescence, since recomplementation of EYFP occurs only within wt/mutant heterodimers. The G52V/wt heterodimer BiFC complex is found only in the rER. As shown in the merged image, after antibody staining this compartment shows a more greenish colour, rather than a complete yellow/orange colour, which would be the case if the antibody were to bind to the rER localised heterodimer. The incomplete green colour of the rER after SN6 staining is most likely the result of antibody detectable premature endoglinwt homodimers in the rER. In contrast, the rER retained G413V/wt heterodimer is detected by the antibody in the rER, as seen in the merged image (rER appears in yellow). Membrane (PM) localized S480C homo- and S480C/wild-type heterodimers are also readily detected by the SN6 antibody.