Figure 7. Relative membrane levels of endogenous endoglin in HMEC-1 cells upon transient or stable mutant protein expression.
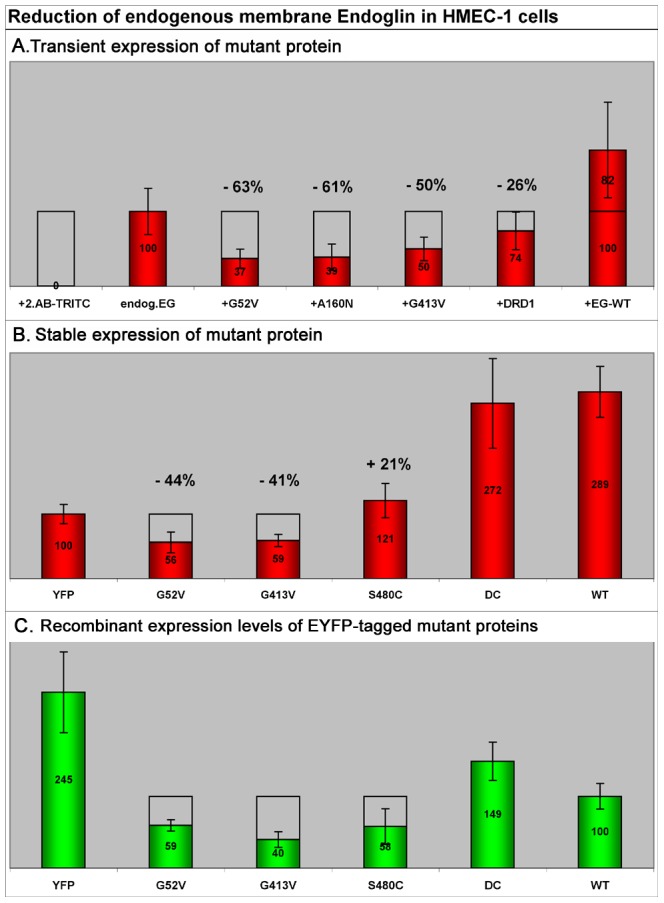
For quantitative measurements of endogenous surface endoglin, cells were fixed, immunostained with mAB SN6 (TRITC), and analysed by fluorescence microscopy. To exclusively stain surface endoglin, the cells were not permeabilized. A. Relative quantification of membrane endoglin after 24 hours of transient expression of mutants and the DRD1 receptor. In comparison to non-transfected HMEC-1 control cells, mutant expression resulted in a total reduction of endogenous membrane endoglin by 50–60%. Expression of the DRD1 receptor decreased the membrane endoglin level by 26%. B. HMEC-1 cells were stably transfected with different EYFP-tagged endoglin mutants and for control comparison also with endoglinwt, endoglinΔC (DC, without cytoplasmic domain), and just EYFP. Stabilized cells were FACS sorted for selection of endoglin variant expressing cells. Subsequently, cells were fixed, immunostained with mAB SN6, and levels of surface endoglin were measured by fluorescence microscopy. In comparison to only EYFP expressing control cells, the rER trapped missense mutants G52V and G413V reduced membrane levels of endogenous endoglin by ∼40%. Expression of the membrane localized mutant S480C raised the level of SN6-detectable molecules in the membrane by 21%. In contrast, additional expression of endoglinwt-EYFP and endoglinΔC-EYFP raised the membrane levels of SN6-detectable molecules by ∼180%. C. Expression levels of EYFP-tagged stably expressed recombinant proteins. Values were normalized to the amount of recombinant expressed endoglinwt (100%). The amount of the different endoglin mutants was only ∼55% of that of endoglinwt. During cell stabilization, only comparably low expression levels of all missense proteins seem to have been tolerated. Cells expressing the variant endoglinΔC (DC, without cytoplasmic domain) showed a 1.5 times higher ectopic expression than endoglinwt.
