Figure 5. NAD-independent D-lactate dehydrogenase (D-iLDH) and NAD-dependent L-lactate dehydrogenase (L-nLDH) activities in cell extracts of H. pylori 26695 wild type and isogenic mutants.
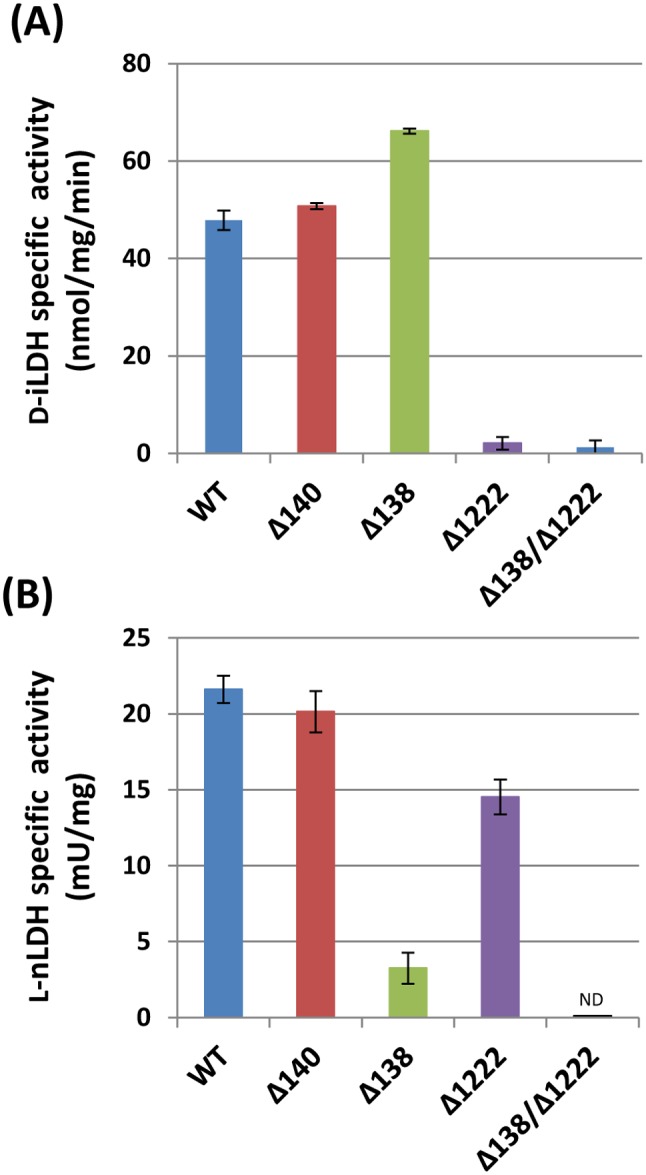
(A) D-iLDH activity was measured as described above. (B) L-nLDH activity was measured by monitoring the reduction of NAD to NADH, arising from the conversion of L-lactate to pyruvate, which was spectrophotometrically detected at 450 nm, and was calibrated based on enclosed NADH standards. All the data represent the means ± SD of three independent experiments, and one unit of the activity is defined as the amount of enzyme that catalyzes the conversion of lactate into pyruvate to generate 1.0 µmole of NADH per minute at 37°C.
