TABLE I.
Characteristics of 476 trials
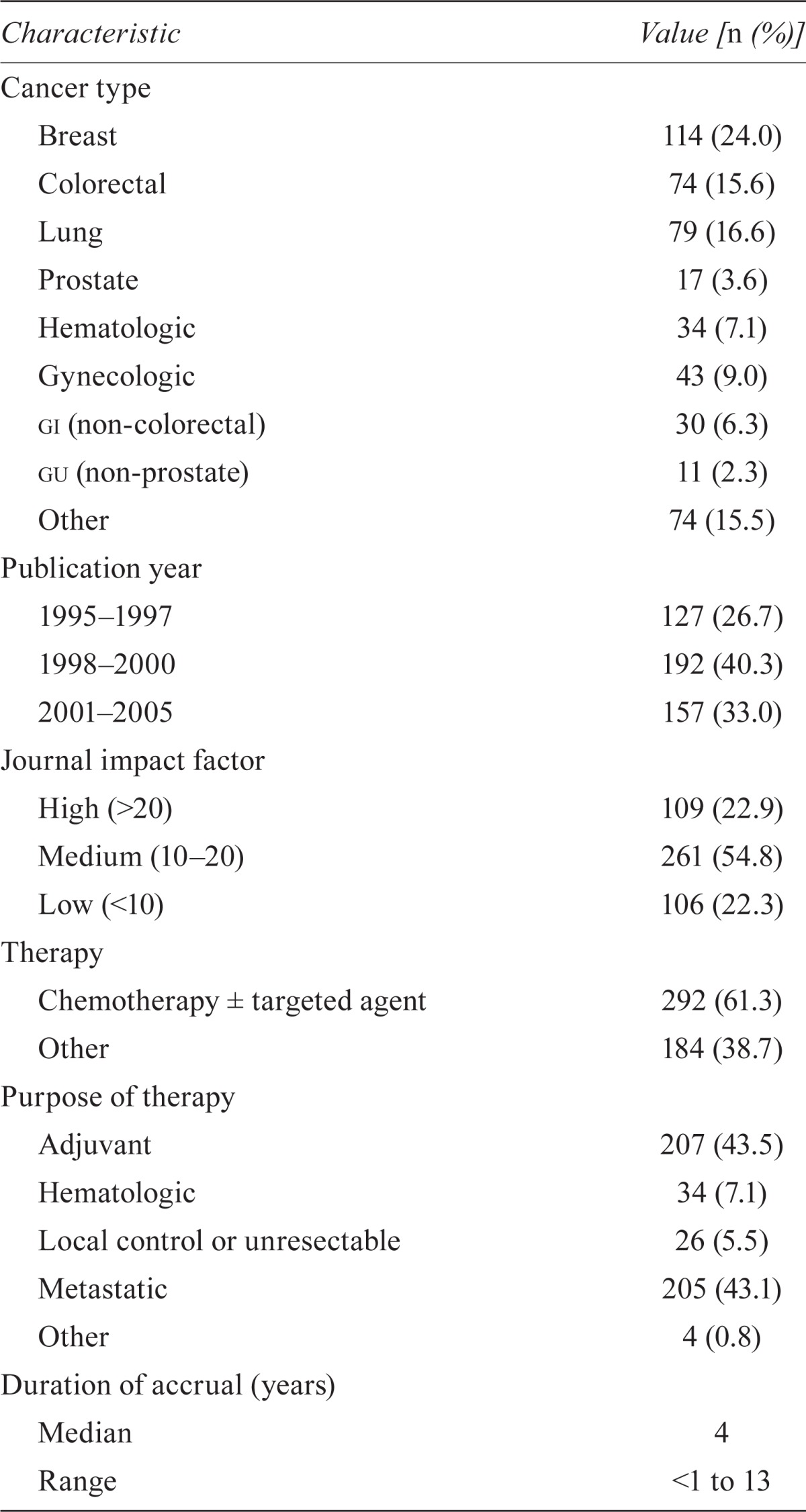
| Characteristic | Value [n (%)] |
|---|---|
| Cancer type | |
| Breast | 114 (24.0) |
| Colorectal | 74 (15.6) |
| Lung | 79 (16.6) |
| Prostate | 17 (3.6) |
| Hematologic | 34 (7.1) |
| Gynecologic | 43 (9.0) |
| gi (non-colorectal) | 30 (6.3) |
| gu (non-prostate) | 11 (2.3) |
| Other | 74 (15.5) |
| Publication year | |
| 1995–1997 | 127 (26.7) |
| 1998–2000 | 192 (40.3) |
| 2001–2005 | 157 (33.0) |
| Journal impact factor | |
| High (>20) | 109 (22.9) |
| Medium (10–20) | 261 (54.8) |
| Low (<10) | 106 (22.3) |
| Therapy | |
| Chemotherapy ± targeted agent | 292 (61.3) |
| Other | 184 (38.7) |
| Purpose of therapy | |
| Adjuvant | 207 (43.5) |
| Hematologic | 34 (7.1) |
| Local control or unresectable | 26 (5.5) |
| Metastatic | 205 (43.1) |
| Other | 4 (0.8) |
| Duration of accrual (years) | |
| Median | 4 |
| Range | <1 to 13 |
| Outcome | |
| Positive | 162 (34.0) |
| Negative | 283 (59.5) |
| Noninferiority | 29 (6.1) |
| Undetermined | 2 (0.4) |
| Independent response review | |
| Yes | 138 (29.0) |
| No | 338 (71.0) |
| Funding | |
| Industry | 183 (38.5) |
| Other | 177 (37.2) |
| Not stated | 116 (24.4) |
| Sample size calculation | |
| Yes | 406 (85.3) |
| No | 70 (14.7) |
| Blinding | |
| Yes | 44 (9.2) |
| No | 432 (90.8) |
| Analysis method | |
| Intention-to-treat | 360 (75.6) |
| Treatment received | 6 (1.3) |
| Not stated | 110 (23.1) |
| Primary endpoint | |
| Overall survival | 221 (46.4) |
| Other | 219 (46.0) |
| Not stated | 36 (7.6) |
| Region | |
| North America | 132 (27.7) |
| Europe | 252 (52.9) |
| International | 67 (14.1) |
| Other | 25 (5.3) |
| Time to publication (years) | |
| Median | 4 |
| Range | 1–18 |
gi = gastrointestinal; gu = genitourinary.
