Abstract
Background
Chemotherapy is an effective treatment in the fight against many cancers. Medication errors in oncology can be particularly serious given the narrow therapeutic window of antineoplastic drugs and their high toxicities. Computerized prescriber order entry (cpoe) has consistently been shown to reduce medication errors and adverse drug events in various settings, but its use in the oncology setting has not been well established. To gain a better understanding of the meaningful use of cpoe systems in the outpatient chemotherapy setting, we undertook a systematic review of systemic therapy cpoe.
Methods
A province-wide expert panel consisting of clinical experts, health information professionals, and specialists in human factors design provided guidance in the development of the research questions, search terms, databases, and inclusion criteria. The systematic review was undertaken by a core team consisting of a medical oncologist, nurse, pharmacist, and methodologist. The medline, embase, cinahl, and compendex databases were searched for relevant evidence.
Results
The database searches resulted in 5642 hits, of which 9 met the inclusion criteria and were retained. In the oncology setting, cpoe systems generally reduce chemotherapy medication errors; however, specific types of errors increase with the use of cpoe. These systems affect practice both positively and negatively with respect to time, workload, and productivity.
Conclusions
Despite the paucity of oncology-specific research, cpoe should be used in outpatient chemotherapy delivery to reduce chemotherapy-related medication errors. Adoption by clinicians will be enhanced by cpoe processes that complement current practice and workflow processes.
Keywords: Computerized prescriber order entry, outpatients, systematic review
1. BACKGROUND
Chemotherapy is an important type of treatment in the fight against many cancers. The very nature of chemotherapy’s toxicity profile also creates potential for harm—and possibly death—if chemotherapy is not ordered, prepared, dispensed, and administered cautiously by members of the health care team.
Medication errors can occur anywhere during the medication process from ordering to administration, and errors can compromise patient safety1,2. A Canadian study3 estimated that 7.5% of patients admitted to acute care hospitals in Canada in 2000 experienced at least 1 adverse event. Drug-related adverse events were the second most common type of event, accounting for approximately 24% of all adverse events.
Medication errors in oncology can be particularly serious, given the narrow therapeutic window and the high toxicities of antineoplastic drugs4,5. In a study conducted by Leape et al.6 of adverse drug events, 39% of errors occurred in the physician order phase, with drug dosing accounting for 28% of all errors. Specific to chemotherapy, Gandhi et al.7 revealed that the most common source of error was within the order phase and that, compared with non-chemotherapy medication errors, chemotherapy errors were 48% more likely to be serious in nature. A study of outpatient care in the oncology setting found that 7% of adult visits and 19% of pediatric visits were associated with a medication error, either in the clinic or at home8. Serious consequences can follow from even a small difference in the intended dose: overdosing can result in considerably more toxicity than usual, and underdosing can result in an unfavourable therapeutic outcome5.
With the introduction of health information technology systems such as systemic treatment computerized prescriber order entry (st cpoe), the link between meaningful use and meaningful benefit has to be carefully considered9. The overarching goals of meaningful use are to enhance practice efficiencies and to improve patient outcomes by optimizing the use of health information technology10. Computerized prescriber order entry has consistently been shown to reduce medication errors and adverse drug events in various settings11–14, but its use in the oncology setting has not been as well established. A systematic review of the cpoe literature specifically within the ambulatory oncology setting was therefore warranted. The focus on the ambulatory oncology setting was chosen because that setting represents more than 90% of all chemotherapy administered. The present systematic review and evidence summary was designed to cover many aspects of st cpoe, including medication error reduction, medication error generation, effects on practice, implementation strategies, and clinical decision supports.
Within Ontario, the implementation of st cpoe systems has grown by 23% since 2004, with 75.5% of all outpatient parenteral chemotherapy visits in 2010 being supported with some type of st cpoe system15. To support this growing need, Cancer Care Ontario’s clinically-driven Systemic Treatment Information Program provides information management, information technology, and e-health solutions to improve the quality, safety, and efficiency of systemic treatment across Ontario. In accordance with meaningful-use goals, the vision of the Systemic Treatment Information Program for improved patient safety is to reach the goal of having 90% of all parenteral systemic treatment (outpatient) visits supported by st cpoe across Ontario. That vision was the nexus for undertaking a systematic review of the topic to evaluate the costs and return on investment associated with such technology solutions. The aim of the present review was therefore to gain a better understanding of the meaningful use of cpoe systems in the outpatient chemotherapy setting and to promote evidence-based practice in the meaningful use of st cpoe systems regardless of the given software application.
2. METHODS
The practice guidelines development cycle16 was used to guide the review, and the systematic review was used as the core methodology to develop the evidentiary base. An expert panel representing various specialty areas such as clinical oncology, pharmacy, nursing, and human factors design provided guidance in the development of the research questions, search terms, databases, and inclusion criteria.
2.1. Literature Search Strategy and Study Selection Criteria
The medline (1996 through November, week 3, 2011), embase (1996 through week 46, 2011), cinahl (1982 through November 24, 2011), and compendex (1969 through November 24, 2011) databases were searched for relevant evidence. The full literature search strategies are set out in Table i. Articles were included in the review if they were published English-language reports of cpoe in the oncology setting for medication error reduction and if they generated outcomes (for example, effects on practice, implementation strategies). Research methodology was restricted to phase ii or iii randomized controlled trials, other comparative studies, single-arm studies, practice guidelines, and systematic reviews for all outcomes, and also qualitative studies for effects on practice, implementation strategies, and clinical decision support outcomes. Evidence was selected and initially reviewed by one author (RC), with other authors reviewing selected abstracts and articles as required.
TABLE I.
Literature search strategy
| Database | |||
|---|---|---|---|
|
| |||
| medline | embase | cinahl | compendex |
| exp Medical Order Entry Systems/ | exp computerized provider order entry/ | 1.TX computerized physician order entry OR TX computerized prescriber entry OR TX computerized provider entry OR TX medication order entry OR TX cpoe or TX moe OR TX computer assisted drug therapy. |
computerized physician order entry OR computerized prescriber order entry OR computerized provider order entry OR medication order entry OR cpoe. |
| exp Drug Therapy, Computer-Assisted/ | computerized physician order entry.mp. | ||
| computerized physician order entry.mp. | computerized prescriber order entry.mp. | ||
| computerized prescriber order entry.mp. | cpoe.mp. | ||
| computerized provider order entry.mp. | MOE.mp | ||
| cpoe.mp. | medication order entry.mp. | ||
| or/1–6 | exp computer assisted drug therapy/ | ||
| limit 7 to english language | or/1–7 | ||
| limit 8 to english language | |||
2.2. Data Extraction
All data were extracted by the methodologist (RC) and later confirmed by an independent data audit. In the data audit, a student uninvolved in any portion of document development independently read the included studies and verified all the data extracted by the methodologist.
3. RESULTS
3.1. Literature Search Results
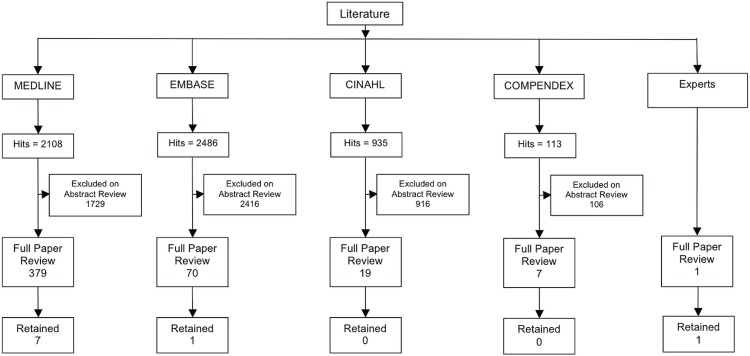
The searches of the selected databases yielded a total of 5642 hits. An initial review by RC of titles and abstracts against the inclusion and exclusion criteria resulted in the identification of 475 papers that were selected for retrieval and full review. The papers were randomly divided into two groups and reviewed against the criteria, with VK and SL reviewing one group, with RC and AC reviewing the other group. That approach allowed for a comparison of the reviews and the identification of papers for inclusion in the systematic review. Suggestions from the expert panel yielded one paper, which was retained. In total, seven unique quantitative and two unique qualitative papers met the eligibility criteria for the systematic review (Figure 1). The research designs described in the quantitative studies included 2-arm trials17–20 and pre–post study designs21–23. The subsection that follows describes the outcomes of the review as they relate to the original research questions. The studies and their results are not mutually exclusive to the distinct research questions (as depicted in Table ii).
Figure 1.
Flow diagram of literature search results.
TABLE II.
Studies selected for inclusion, by question (not mutually exclusive)
| Question or topic | Selected articles | |||
|---|---|---|---|---|
| Quantitative | Qualitative | |||
|
|
|
|||
| (n) | References | (n) | References | |
| Medication error reduction | 5 | Huertas Fernandez et al., 200617 | na | — |
| Kim et al., 200621 | ||||
| Voeffray et al., 200622 | ||||
| Small et al., 200818 | ||||
| Collins and Elsaid, 201123 | ||||
| Medication error generation | 4 | Beer et al., 200219 | na | — |
| Kim et al., 200621 | ||||
| Small et al., 200818 | ||||
| Collins and Elsaid, 201123 | ||||
| Impact of st cpoe on practice | 2 | Beer et al., 200219 | 1 | Kozakiewicz et al., 200524 |
| Khajouei et al., 201020 | ||||
| Implementation strategies | 0 | — | 1 | Greenberg et al., 200625 |
| Clinical decision supports | 0 | — | 0 | — |
na = none available; st cpoe = systemic therapy computerized prescriber order entry.
3.2. The Impact of ST CPOE on Medication Errors
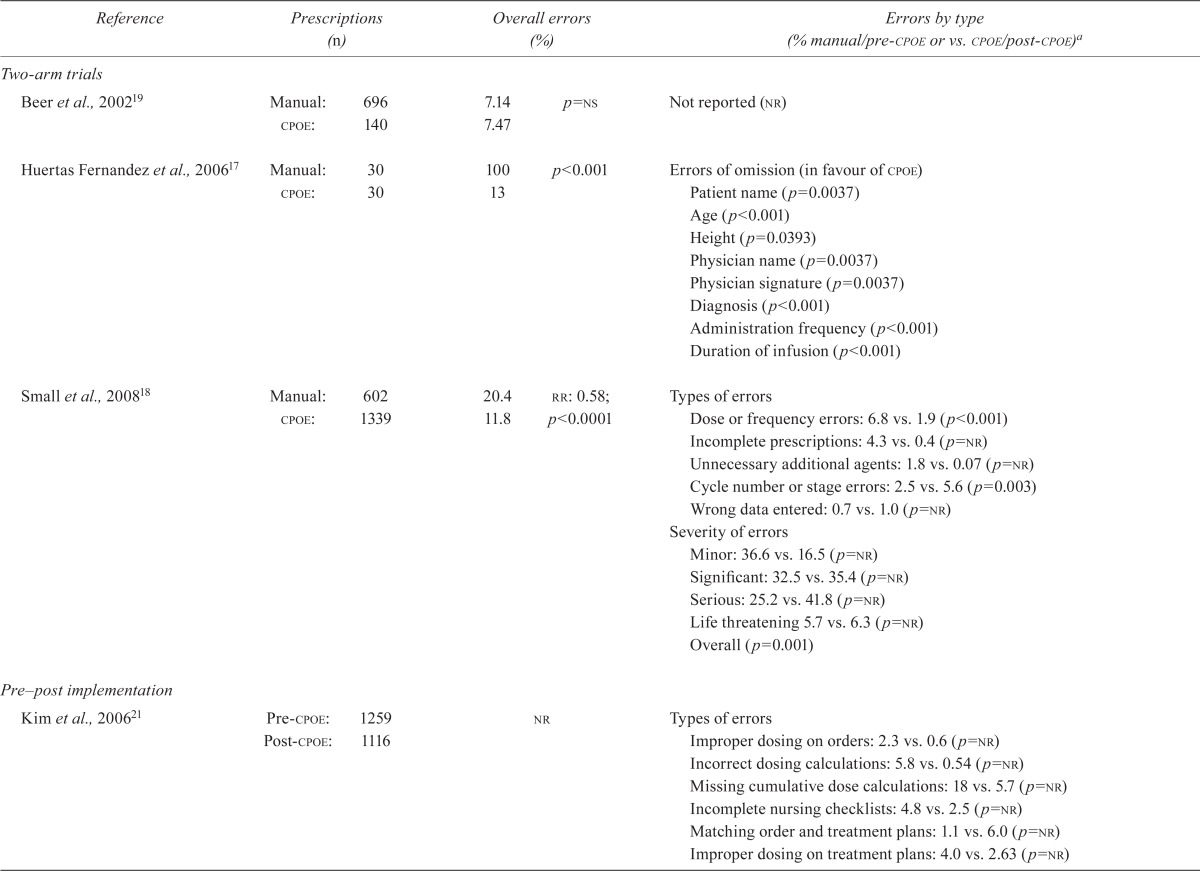
Five studies17,18,21–23 demonstrating that cpoe reduces chemotherapy medication errors in the adult outpatient setting were identified. All reported error reduction for at least some types of errors. Huertas– Fernandez et al.17 compared manual and computerized prescriptions during 1 month in the medical oncology department of a university hospital. The chance of at least 1 error was 100% in a manual prescription compared with 13% in a computerized prescription (p < 0.001). The median number of errors in manual compared with computerized prescriptions was 5 compared with 0 (p < 0.001). The most common errors were errors of omission in manual compared with computerized prescriptions, including patient name (p = 0.0037), age (p < 0.001), and height (p = 0.0393); physician name (p = 0.0037) and signature (p < 0.001); diagnosis (p < 0.001); administration frequency (p < 0.001); and duration of infusion (p < 0.001).
In a much larger study, Small et al.18 also compared manual and computerized complex chemo-therapy prescriptions. The error rate was 20.4% in manual orders; in computerized orders, it was 11.8%. That difference represents an overall reduction in the relative risk (rr) for errors of 42% [rr: 0.58; 95% confidence interval (ci): 0.47 to 0.72; p < 0.0001]. Moreover, the types of errors differed significantly according to the prescription method (p < 0.001), with cpoe being associated with fewer dose or frequency errors, incomplete prescriptions, and unnecessary additional agents. As a proportion of total errors, minor errors were fewer with computerized prescribing [16.5% vs. 36.6% with manual prescribing, p = not reported (nr)]. Overall, the severity of errors differed significantly by prescribing method (p = 0.001).
Voeffray et al.22 evaluated prescribing errors for 15 months before and 21 months after cpoe implementation. The error rate was 15% pre-cpoe and 5% post-cpoe, with 92% of the post-cpoe errors being found in handwritten prescriptions. Pre-cpoe, 19% of errors were major, and 81% of errors were minor; post-cpoe, all errors were minor. The monthly average error rate was reported to be 13.1% pre-cpoe and 0.6% post-cpoe, representing a reduction to less than one twentieth of the original error rate.
Kim et al.21 evaluated cpoe in the pediatric setting using a pre–post cpoe implementation design. Compared with manual prescribing, cpoe resulted in fewer errors of improper dosing on orders (2.3% vs. 0.6%; rr: 0.26; 95% ci: 0.11 to 0.61), incorrect dosing calculations (5.8% vs. 0.54%; rr: 0.09; 95% ci: 0.03 to 0.34), missing cumulative dose calculations (18% vs. 5.7%; rr: 0.32; 95% ci: 0.14 to 0.77), and incomplete nursing checklists (4.8% vs. 2.5%; rr: 0.51; 95% ci: 0.36 to 0.80). No difference with respect to improper dosing on treatment plans was observed (4.0% vs. 2.6%; rr: 0.66; 95% ci: 0.42 to 1.04). Unfortunately, p values were not provided for any of the reported error types.
Collins and Elsaid23 reported on prescribing errors for oral chemotherapy in an inpatient setting for a 24-month period before cpoe implementation and 6 months after implementation. The implementation of cpoe significantly reduced the risk of prescribing errors by 69% (odds ratio: 0.31; 95% ci: 0.11 to 0.86; p = 0.023). Table iii summarizes error rates for manual and cpoe prescribing systems in the oncology setting.
TABLE III.
Error rates for manual and computerized prescriber order entry (cpoe) prescribing systems in the oncology setting
| Reference | Prescriptions (n) | Overall errors (%) | Errors by type (% manual/pre-cpoe or vs. cpoe/post-cpoe)a | ||
|---|---|---|---|---|---|
| Two-arm trials | |||||
| Beer et al., 200219 | Manual: | 696 | 7.14 | p=ns | Not reported (nr) |
| cpoe: | 140 | 7.47 | |||
| Huertas Fernandez et al., 200617 | Manual: | 30 | 100 | p<0.001 | Errors of omission (in favour of cpoe) |
| cpoe: | 30 | 13 | Patient name (p=0.0037) | ||
| Age (p<0.001) | |||||
| Height (p=0.0393) | |||||
| Physician name (p=0.0037) | |||||
| Physician signature (p=0.0037) | |||||
| Diagnosis (p<0.001) | |||||
| Administration frequency (p<0.001) | |||||
| Duration of infusion (p<0.001) | |||||
| Small et al., 200818 | Manual: | 602 | 20.4 | rr: 0.58; | Types of errors |
| cpoe: | 1339 | 11.8 | p<0.0001 | Dose or frequency errors: 6.8 vs. 1.9 (p<0.001) | |
| Incomplete prescriptions: 4.3 vs. 0.4 (p=nr) | |||||
| Unnecessary additional agents: 1.8 vs. 0.07 (p=nr) | |||||
| Cycle number or stage errors: 2.5 vs. 5.6 (p=0.003) | |||||
| Wrong data entered: 0.7 vs. 1.0 (p=nr) | |||||
| Severity of errors | |||||
| Minor: 36.6 vs. 16.5 (p=nr) | |||||
| Significant: 32.5 vs. 35.4(p=nr) | |||||
| Serious: 25.2 vs. 41.8 (p=nr) | |||||
| Life threatening 5.7 vs. 6.3 (p=nr) | |||||
| Overall (p=0.001) | |||||
| Pre–post implementation | |||||
| Kim et al., 200621 | Pre-cpoe: | 1259 | nr | Types of errors | |
| Post-cpoe: | 1116 | Improper dosing on orders: 2.3 vs. 0.6 (p=nr) | |||
| Incorrect dosing calculations: 5.8 vs. 0.54 (p=nr) | |||||
| Missing cumulative dose calculations: 18 vs. 5.7 (p=nr) | |||||
| Incomplete nursing checklists: 4.8 vs. 2.5 (p=nr) | |||||
| Matching order and treatment plans: 1.1 vs. 6.0 (p=nr) | |||||
| Improper dosing on treatment plans: 4.0 vs. 2.63 (p=nr) | |||||
| Pre–post implementation | |||||
| Voeffray et al., 200622 | Pre-cpoe: | 940 | 15 | Minor errors: 81 vs. 100 | |
| Post-cpoe: | 1505 | 5 | Major errors: 19 vs. 0 | ||
| Collins and Elsaid, 201123 | Pre-cpoe: | 412 | Reduction in prescribing errors with cpoe (or: 0.31; 95% ci: 0.11 to 0.89; p=0.023) | Errors in clinical decision making | |
| Post-cpoe: | 126 | Wrong dosing schedule or duration: 3.2 vs. 0.0 (p=nr) | |||
| Dose likely leading to high serum levels: 0.7 vs. 0.8 (p=nr) | |||||
| Dose likely leading to low serum levels: 0.5 vs. 0.8 (p=nr) | |||||
| Dose exceeding maximum range for the indication: 0.5 vs. 0.0 (p=nr) | |||||
| Errors in transcription | |||||
| Omitted or unclear drug name, route of administration: 0.2 vs. 0.0 (p=nr) | |||||
| Errors related to prescribing policy | |||||
| Prescribing policy not followed: 3.6 vs. 1.6 (p=nr) | |||||
rr= relative risk; or= odds ratio; ci= confidence interval.
Boldface type signals errors that increased after cpoe implementation.
3.3. Generation of New Errors
Four studies18,19,21,23 demonstrating that cpoe might increase chemotherapy medication errors were identified. Small et al.18 reported that error types differed significantly by the prescription method (p < 0.001). Computerized prescribing was associated with more frequent cycle number or stage errors and instances of wrong data entered (for example, height, weight). These authors also categorized each prescribing error according to severity, with serious errors defined as those that might cause either harm or significant undertreatment. Life-threatening errors were defined as those possibly resulting in death. Compared with manual prescribing, cpoe was more frequently associated with serious (25.2% vs. 41.8%, p = nr), significant (32.5% vs. 35.4%, p = nr), and life-threatening errors (5.7% vs. 6.3%, p = nr).
Beer et al.19 took a different approach, in that they measured the pharmacist intervention rate, which was defined as any problem with a medication order that required physician clarification before the pharmacist could process the order. They observed no statistically significant difference in the intervention rate for manual and computerized orders (7.14% vs. 7.47%, p = nonsignificant, Table iii). Neither Small et al.18 nor Beer et al.19 referred to specific prescriber or system features that might have contributed to the increase in errors or interventions.
Kim et al.21 evaluated cpoe in the pediatric setting using a pre–post cpoe implementation design. Compared with manual prescribing, cpoe resulted in more errors for matching order and treatment plans (1.1% vs. 6.0%; rr: 5.4; 95% ci: 3.1 to 9.5), although it is unknown if that result was statistically significant.
Collins and Elsaid23 reported on prescribing errors for inpatient oral chemotherapy during a 24-month period before cpoe implementation and 6 months after implementation. After cpoe implementation, more dosing errors likely leading to high (0.7% vs. 0.8%) or low serum levels (0.5% vs. 0.8%) were observed. The significance levels for the errors were not reported.
3.4. Effect of ST CPOE on Clinician Practice
Beer et al.19 evaluated the effect of cpoe on pharmacy practice. Pharmacist intervention rates were measured, as was the time the pharmacist needed to review each order. All medications listed on a given prescription were considered to be a single order regardless of how many were listed. The time to review each order was measured. If a pharmacist intervention was needed to complete the order review, the timing of the review process continued throughout the duration of the pharmacist intervention. The mean time to complete a prescription order review was significantly longer for a computerized prescription than for a manual prescription (11.1 minutes vs. 5.96 minutes, p < 0.001). Even when categorized by orders that required an intervention (18.32 vs. 13.49, p < 0.001) and those that did not (10.56 vs. 5.35, p < 0.0001), significantly more pharmacist review time was required for computerized prescriptions than for manual prescriptions.
Khajouei et al.20 compared the effect of predefined order sets on the efficiency of chemotherapy prescribing within a cpoe system. In a counterbalanced design, 10 hematology or oncology physicians were provided a clinical scenario and asked to order medications using and not using a predefined order set. Optimally, the predefined order set scenario required 61 keystrokes and mouse clicks, and the non-order set scenario required 86 (p < 0.01).
One qualitative study pertaining to the impact of cpoe on oncology practice was identified24. In a failure mode and effects analysis conducted with a multidisciplinary team, the team was able to affect practice by developing a uniform and safe chemo-therapy ordering system.
3.5. Strategies to Enhance Implementation of ST CPOE
Based on the implementation of a st cpoe system in 11 organizations across Ontario, Greenberg et al.25 identified several key components to successful implementation, including having a fully staffed project team; enlisting the support of clinical and administrative leadership; involving stakeholders in decision-making to ensure a sense of ownership and empowerment; providing in-depth, on-site training; testing the system extensively; and providing ongoing customized support and maintenance.
4. DISCUSSION
Patient safety has garnered much attention, particularly since the 1999 report from the U.S. Institute of Medicine26 which estimated that, in the United States alone, 80,000 people are hospitalized and 7000 die every year because of medication errors in the inpatient setting, many of which are preventable. Computerized prescriber order entry is one promising technology for the reduction of medication errors in the inpatient and outpatient settings alike.
Medication errors in the oncology setting can be particularly serious, given the toxicity of chemotherapeutic agents. The results of the present systematic review clearly demonstrated the paucity of oncology-specific cpoe literature, but the studies cited here are consistent with the existing systematic reviews describing the benefits of cpoe systems14,27 and the unanticipated outcomes28. The few studies included in this review demonstrate that cpoe in the oncology setting does contribute to a decline in the incidence of medication errors17,18,21–23, as it does, in some instances, to the potential for increased errors18,19,21,23. The cpoe system, clinical decision supports, and associated interface elements must be carefully designed to optimize the benefits of st cpoe systems.
Systemic therapy cpoe can also affect practice, particularly workflow, communication between health care professionals, and communication between health care professionals and patients. Unfortunately, the study results are not consistent, probably reflecting the true nature of variability in the practice setting. Several non-oncology studies have also found that eprescribing has had a negative effect on workflow in terms of time and workload27,29,30,31, but other studies report a positive effect with respect to time and workload32–35, productivity32, and communications33,36. One study reports both positive and negative effects on various aspects of time and workload37. The results of qualitative studies in oncology and non-oncology settings38–40 resemble the empirical evidence. The totality of the evidence reveals that, as with any new technology, cpoe will have both positive and negative effects on practice.
Only a handful of studies have evaluated cpoe implementation, either empirically or qualitatively, in the outpatient setting, and none were in the oncology setting. Empirical non-oncology studies all look at very different factors that might affect implementation, including the use of a cpoe “champion”41; respondent use of a home computer for work42; and physician, structural, and cultural variables43. Some common themes emerging from those studies are the needs for a strong vision and motivation for introducing cpoe; for the involvement of stakeholders in decision-making; for the provision of in-depth, on-site, and ongoing training before and after launch; and for the establishment of mechanisms to efficiently respond to problems identified by end users26,41,44–46.
5. CONCLUSIONS
Computerized prescriber order entry is a promising technology for the reduction of medication errors and the potential adverse drug events associated with those medication errors. Based on a review of the literature, the following 6 strategies are presented:
cpoe systems should be used in outpatient chemo-therapy delivery to reduce chemotherapy-related medication errors.
Clinical, technical, and leadership champions are vital to support the successful adoption of cpoe within an organization.
A multidisciplinary team approach should be used in the design, selection, workflow evaluation, implementation or evaluation (or both), and ongoing monitoring of the cpoe system.
To enhance adoption by clinicians, cpoe processes that complement current practice and workflow processes should be ensured.
cpoe systems, clinical decision supports, and associated interface elements must be carefully designed to reduce the potential for error.
Development and implementation of a risk-assessment process to identify actual or potential unanticipated consequences and the generation of new errors are warranted, as is the development of strategies to modify the system accordingly.
The meaningful-use campaign emphasizes the need to use technology in a meaningful manner to improve the quality of care and to be able to measure the effectiveness of the technology solution. When optimally designed and implemented, st cpoe systems can contribute to achieving the goals of meaningful use—specifically, improvement in quality, safety, and efficiency, and enhancement of care coordination. The paucity of st cpoe research in the oncology setting warrants consideration, because the current literature is limited with respect to measuring the effectiveness of such systems in providing the desired clinical outcomes beyond error reduction, a topic that will need to be investigated in future research. Other considerations, such as a standardized design and architecture for these systems to correlate with best clinical practice, would be beneficial. Meaningful use is optimized by quality measurement—in this case, by focusing on the clinical processes within which these systems are used. Measures could include utilization rates, triggered alert rates (in parallel with override rates), and clinically important metrics such as adverse-event and near-miss rates. Lastly, cpoe systems are moving toward becoming integrated special-application modules within complex medication management systems, with associated processes such as e-prescribing. Careful analysis of error reduction, effects on practice, and generation of new errors will need to be considered.
6. ACKNOWLEDGMENTS
The Program in Evidence-Based Care is supported by the Ontario Ministry of Health and Long-Term Care through Cancer Care Ontario. All work produced by the Program in Evidence-Based Care is editorially independent from the Ministry.
This work was previously presented at the World Cancer Conference of the Union for International Cancer Control; Montreal, QC; August 27–30, 2012; the National Oncology Pharmacy Symposium; Saskatoon, SK; October 25–28, 2012; and the American Society of Clinical Oncology Quality Care Symposium; San Diego, CA, U.S.A.; November 30–December 1, 2012.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Berman A. Reducing medication errors through naming, labeling, and packaging. J Med Syst. 2004;28:9–29. doi: 10.1023/B:JOMS.0000021518.60670.10. [DOI] [PubMed] [Google Scholar]
- 2.Hellier E, Edworthy J, Derbyshire N, Costello A. Considering the impact of medicine label design characteristics on patient safety. Ergonomics. 2006;49:617–30. doi: 10.1080/00140130600568980. [DOI] [PubMed] [Google Scholar]
- 3.Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170:1678–86. doi: 10.1503/cmaj.1040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MR, Anderson RW, Attilio RM, Green L, Muller RJ, Pruemer JM. Preventing medication errors in cancer chemo-therapy. Am J Health Syst Pharm. 1996;53:737–46. doi: 10.1093/ajhp/53.7.737. [DOI] [PubMed] [Google Scholar]
- 5.Kohler DR, Montello MJ, Green L, et al. Standardizing the expression and nomenclature of cancer treatment regimens. American Society of Health-System Pharmacist (ashp), American Medical Association (ama), American Nurses Association (ana) Am J Health Syst Pharm. 1998;55:137–44. doi: 10.1093/ajhp/55.2.137. [DOI] [PubMed] [Google Scholar]
- 6.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274:35–43. doi: 10.1001/jama.1995.03530010049034. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi TK, Bartel SB, Shulman LN, et al. Medication safety in the ambulatory chemotherapy setting. Cancer. 2005;104:2477–83. doi: 10.1002/cncr.21442. [DOI] [PubMed] [Google Scholar]
- 8.Walsh KE, Dodd KS, Seetharaman K, et al. Medication errors among adults and children with cancer in the outpatient setting. J Clin Oncol. 2009;27:891–6. doi: 10.1200/JCO.2008.18.6072. [DOI] [PubMed] [Google Scholar]
- 9.Classen C, Bates D, Denham C. Meaningful use of prescriber order entry. J Patient Saf. 2010;6:15–23. doi: 10.1097/PTS.0b013e3181d108db. [DOI] [PubMed] [Google Scholar]
- 10.Yu P. The evolution of oncology electronic health records. Cancer J. 2011;17:197–202. doi: 10.1097/PPO.0b013e3182269629. [DOI] [PubMed] [Google Scholar]
- 11.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–16. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 12.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 13.Shamliyan TA, Duval S, Du J, Kane RL. Just what the doctor ordered. Review of the evidence of the impact of computerized physician order entry system on medication errors. Health Serv Res. 2008;43:32–53. doi: 10.1111/j.1475-6773.2007.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammenwerth E, Schnell–Inderst P, Machan C, Siebert U. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inform Assoc. 2008;15:585–600. doi: 10.1197/jamia.M2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Quality Council of Ontario (cqco) Cancer System Quality Index 2011: Percentage of Systemic Treatment Visits Supports by Computerized Physician Order Entry (cpoe) [Web page] Toronto, ON: CQCO; 2011. [Current version available online at: http://www.csqi.on.ca/cms/One.aspx?portalId=126935&pageId=128022; cited March 4, 2014] [Google Scholar]
- 16.Browman GP, Levine MN, Mohide EA, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 17.Huertas Fernandez MJ, Baena–Cañada JM, Martínez Bautista MJ, Arriola Arellano E, García Palacios MV. Impact of computerised chemotherapy prescriptions on the prevention of medication errors. Clin Transl Oncol. 2006;8:821–5. doi: 10.1007/s12094-006-0138-1. [DOI] [PubMed] [Google Scholar]
- 18.Small MDC, Barrett A, Price GM. The impact of computerized prescribing on error rate in a department of oncology/hematology. J Oncol Pharm Pract. 2008;14:181–87. doi: 10.1177/1078155208094453. [DOI] [PubMed] [Google Scholar]
- 19.Beer J, Dobish R, Chambers C. Physician order entry: a mixed blessing to pharmacy? J Oncol Pharm Pract. 2002;8:119–26. doi: 10.1191/1078155202jp099oa. [DOI] [Google Scholar]
- 20.Khajouei R, Peek N, Wierenga PC, Kersten MJ, Jaspers MW. Effect of predefined order sets and usability problems on efficiency of computerized medication ordering. Int J Med Inform. 2010;79:690–8. doi: 10.1016/j.ijmedinf.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim GR, Chen AR, Arceci RJ, et al. Error reduction in pediatric chemotherapy: computerized order entry and failure modes and effects analysis. Arch Pediatr Adolesc Med. 2006;160:495–8. doi: 10.1001/archpedi.160.5.495. [DOI] [PubMed] [Google Scholar]
- 22.Voeffray M, Pannatier A, Stupp R, Fucina N, Leyvraz S, Wasserfallen JB. Effect of computerisation on the quality and safety of chemotherapy prescription. Qual Saf Health Care. 2006;15:418–21. doi: 10.1136/qshc.2005.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins CM, Elsaid KA. Using an enhanced oral chemotherapy computerized provider order entry system to reduce prescribing errors and improve safety. Int J Qual Health Care. 2011;23:36–43. doi: 10.1093/intqhc/mzq066. [DOI] [PubMed] [Google Scholar]
- 24.Kozakiewicz JM, Benis LJ, Fisher SM, Marseglia JB. Safe chemotherapy administration: using failure mode and effects analysis in computerized prescriber order entry. Am J Health Syst Pharm. 2005;62:1813–16. doi: 10.2146/ajhp040553. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg A, Kramer S, Welch V, O’Sullivan E, Hall S. Cancer Care Ontario’s computerized physician order entry system: a province-wide patient safety innovation. Healthc Q. 2006;9:108–13. doi: 10.12927/hcq.2006.18468. [DOI] [PubMed] [Google Scholar]
- 26.Kohn LT, Corrigan JM, Donaldson MS, editors. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 1999. [PubMed] [Google Scholar]
- 27.Eslami S, Abu-Hanna A, de Keizer NF. Evaluation of outpatient computerized physician medication order entry systems: a systematic review. J Am Med Inform Assoc. 2007;14:400–6. doi: 10.1197/jamia.M2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ash JS, Sittig DF, Dykstra R, Campbell E, Guappone K. The unintended consequences of computerized provider order entry: findings from a mixed methods exploration. Int J Med Inform. 2009;78(suppl 1):S69–76. doi: 10.1016/j.ijmedinf.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollingworth W, Devine EB, Hansen RN, et al. The impact of e-prescribing on prescriber and staff time in ambulatory care clinics: a time motion study. J Am Med Inform Assoc. 2007;14:722–30. doi: 10.1197/jamia.M2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devine EB, Hollingworth W, Hansen RN, et al. Electronic prescribing at the point of care: a time-motion study in the primary care setting. Health Serv Res. 2010;45:152–71. doi: 10.1111/j.1475-6773.2009.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desroches CM, Agarwal R, Angst CM, Fischer MA. Differences between integrated and stand-alone e-prescribing systems have implications for future use. Health Aff (Millwood) 2010;29:2268–77. doi: 10.1377/hlthaff.2010.0557. [DOI] [PubMed] [Google Scholar]
- 32.Wang CJ, Patel MH, Schueth AJ, et al. Perceptions of standards-based electronic prescribing systems as implemented in outpatient primary care: a physician survey. J Am Med Inform Assoc. 2009;16:493–502. doi: 10.1197/jamia.M2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupp MT, Warholak TL. Evaluation of e-prescribing in chain community pharmacy: best-practice recommendations. J Am Pharm Assoc. 2008;48:364–70. doi: 10.1331/JAPhA.2008.07031. [DOI] [PubMed] [Google Scholar]
- 34.Tan WS, Phang JS, Tan LK. Evaluating user satisfaction with an electronic prescription system in a primary care group. Ann Acad Med Singapore. 2009;38:494–7. [PubMed] [Google Scholar]
- 35.Rahimi B, Timpka T. Pharmacists’ views on integrated electronic prescribing systems: associations between usefulness, pharmacological safety, and barriers to technology use. Eur J Clin Pharmacol. 2011;67:179–84. doi: 10.1007/s00228-010-0936-9. [DOI] [PubMed] [Google Scholar]
- 36.Hammar T, Nystrom S, Petersson G, Rydberg T, Astrand B. Swedish pharmacists value eprescribing: a survey of a nationwide implementation. J Pharm Health Serv Res. 2010;1:23–32. [Google Scholar]
- 37.Duffy RL, Yiu SS, Molokhia E, Walker R, Perkins RA. Effects of electronic prescribing on the clinical practice of a family medicine residency. Fam Med. 2010;42:358–63. [PubMed] [Google Scholar]
- 38.Weingart SN, Massagli M, Cyrulik A, et al. Assessing the value of electronic prescribing in ambulatory care: a focus group study. Int J Med Inform. 2009;78:571–8. doi: 10.1016/j.ijmedinf.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal R, Angst CM, DesRoches CM, Fischer MA. Technological viewpoints (frames) about electronic prescribing in physician practices. J Am Med Inform Assoc. 2010;17:425–31. doi: 10.1136/jamia.2009.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapane KL, Rosen RK, Dube C. Perceptions of e-prescribing efficiencies and inefficiencies in ambulatory care. Int J Med Inform. 2011;80:39–46. doi: 10.1016/j.ijmedinf.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pare G, Sicotte C, Jacques H. The effects of creating psychological ownership on physicians’ acceptance of clinical information systems. J Am Med Inform Assoc. 2006;13:197–205. doi: 10.1197/jamia.M1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devine EB, Patel R, Dixon DR, Sullivan SD. Assessing attitudes toward electronic prescribing adoption in primary care: a survey of prescribers and staff. Inform Prim Care. 2010;18:177–87. doi: 10.14236/jhi.v18i3.770. [DOI] [PubMed] [Google Scholar]
- 43.Kralewski JE, Dowd BE, Cole–Adeniyi T, Gans D, Malakar L, Elson B. Factors influencing physician use of clinical electronic information technologies after adoption by their medical group practices. Health Care Manage Rev. 2008;33:361–7. doi: 10.1097/01.HCM.0000318773.67395.ce. [DOI] [PubMed] [Google Scholar]
- 44.Ash JS, Stavri PZ, Kuperman GJ. A consensus statement on considerations for a successful cpoe implementation. J Am Med Inform Assoc. 2003;10:229–34. doi: 10.1197/jamia.M1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crosson JC, Isaacson N, Lancaster D, et al. Variation in electronic prescribing implementation among twelve ambulatory practices. J Gen Intern Med. 2008;23:364–71. doi: 10.1007/s11606-007-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman JM, Baker DK, Howard SC, Laver JH, Shenep JL. Safe and successful implementation of cpoe for chemotherapy at a children’s cancer center. J Natl Compr Canc Netw. 2011;9(suppl 3):S36–50. doi: 10.6004/jnccn.2011.0131. [DOI] [PubMed] [Google Scholar]