Figure 1.
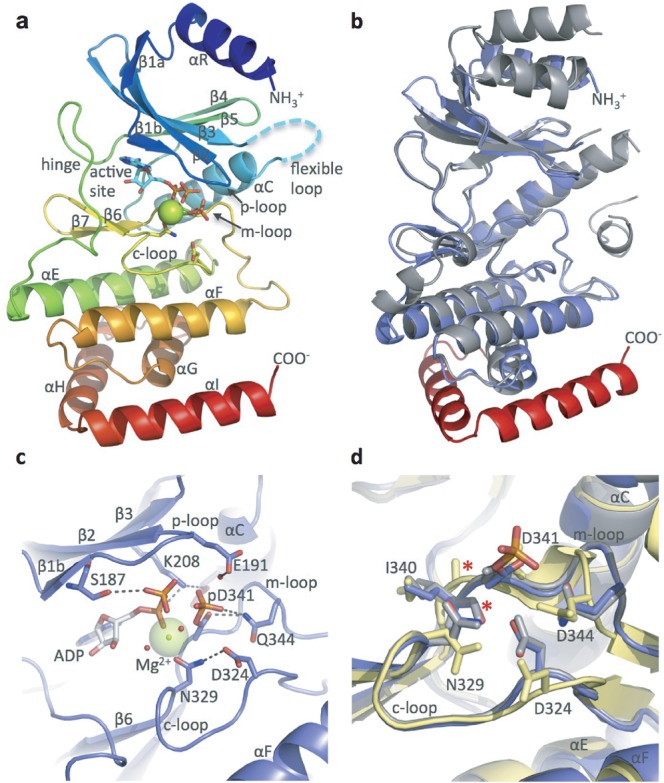
hsRio1 structure. (a) Overall structure of hsRio1 residues 140-430. The entire conserved RIO domain (αR-αG) is observed in the hsRio1 structure (colored blue to red N-terminal to C-terminal. In addition, two helices, αH and αI, C-terminal to the RIO domain are observed. Density is not observed for 32 residues of the flexible region (represented by the cyan dashed line). (b) Alignment of the archaeal Rio1 (gray) with the hsRio1 structure (blue in the RIO domain, red in the extension). (c) Detailed view of the active site and interactions with ADP, Mg2+ and the pAsp (pD341). Invariant residues from the p-, c- and m-loops are shown in sticks. (d) Alignment of the c- and m-loops from the A. fulgidus Rio1/ATP complex (active state; gray); the adenosine complex (inactive state; yellow) and hsRio1/ADP/pAsp (this structure; blue). The red asterisk shows the backbone carbonyl that flips when ATP binds to produce the active conformation. The residues are labeled according to numbering in hsRio1.
