Figure 6.
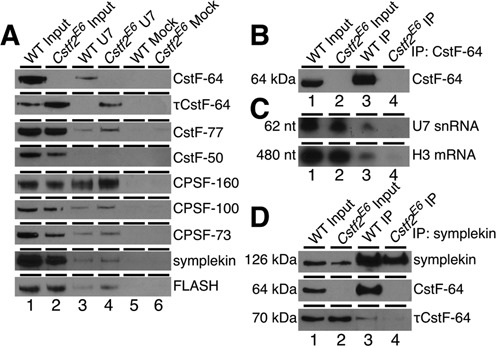
CstF-64 is a component of the replication-dependent histone mRNA 3′ end-processing complex. (A) Western blot analysis of the proteins isolated from a pull-down experiment using anti-U7 snRNP oligonucleotide (lanes 3 and 4) or unrelated mock oligonucleotide (lanes 5 and 6) in WT and Cstf2E6 ESCs nuclear extracts. 1/100th of the nuclear extracts from the wild type ESCs (lane 1) and Cstf2E6 cells (lane 2) before the pull down were also loaded on the gel serving as an input control. Antibodies against the indicated proteins were used. (B) Western blot analysis of immunoprecipitation using an anti-CstF-64 antibody in wild type ECCs (lane 3) and Cstf2E6 (lane 4) cells. 1/100th of the total proteins was also loaded as an input control. Wild type ESCs (lane 1) and Cstf2E6 cells (lane 2). (C) Northern blot of RNA from immunoprecipitation with antibodies against CstF-64 that were hybridized with radiolabeled ribo-probes specific for U7 snRNA or Hist1h3c mRNA. Lane 1, 2 μg of total RNA from wild type ESCs; lane 2, 2 μg of total RNA from Cstf2E6 cells; lanes 3–4, 2 μg of RNA purified from CstF-64 immunoprecipitation from wild type ESCs (lane 3) or Cstf2E6 cells (lane 4). (D) Western blot analysis of immunoprecipitation with an anti-symplekin antibody in wild type (lane 3) and Cstf2E6 (lane 4) ESCs protein lysates. IP precipitates from wild type and Cstf2E6 ESCs were probed for interaction with CstF-64 and τCstF-64 as indicated.
