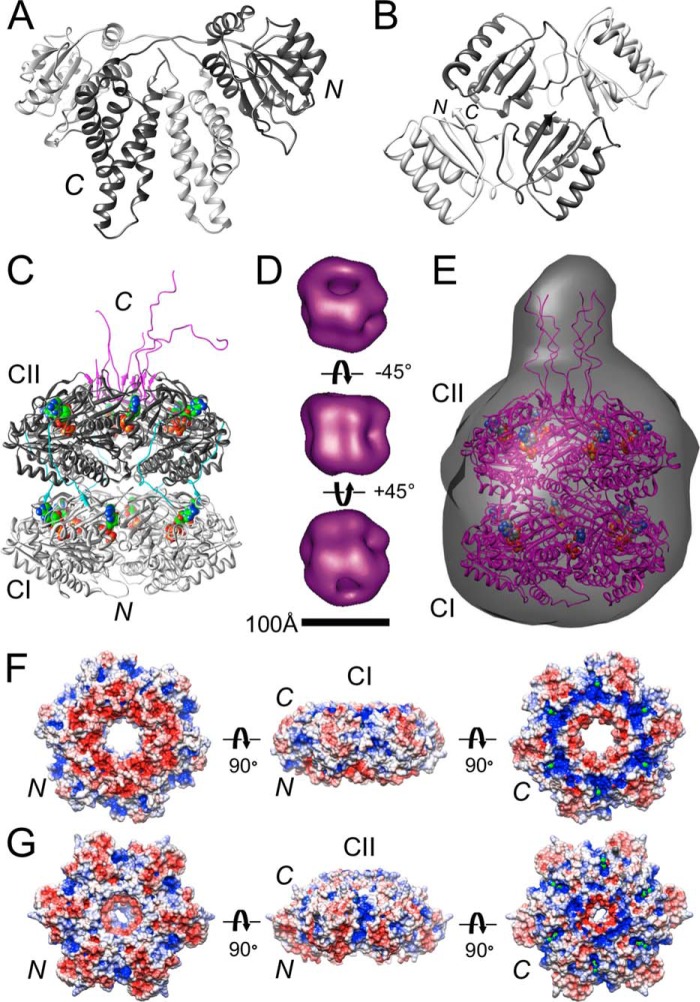
FIGURE 1.
Architecture of S. elongatus Kai proteins. A, crystal structure of domain-swapped KaiA dimer with subunits colored in light and dark gray, respectively (61). B, crystal structure of KaiB tetramer (30). Structures of KaiC at high and low resolution are shown. C, crystal structure of S. elongatus KaiC with the N-terminal CI and C-terminal CII domains colored in light and dark gray, respectively (31, 37). Note the C-terminal tentacles (colored in magenta) constituting the KaiA binding site that are resolved to different degrees at 2.8 Å resolution. ATP molecules are drawn in space-filling mode with carbon atoms highlighted in green. In all depictions of KaiC included here, the original orientation has been retained, i.e. the N-terminal CI ring is at the bottom and the C-terminal CII ring is at the top. D, negative stain EM structure of KaiC viewed from the side (center) and rotated by +45° (bottom) and −45° (top) around the horizontal axis (37). E, SAXS structure of KaiC (34). The protrusion distinguishes the CII from the CI end. ESPs of the CI (F) and CII (G) rings in KaiC hexamer are shown. The minimum and maximum values of the electrostatic potential are −5 (red) and +5 kt/e (blue), respectively. KaiC rings are viewed from the N terminus (left), the side (center), and the C terminus (right). ATP molecules (green) can be seen inside positively polarized clefts in the views from the C-terminal side.