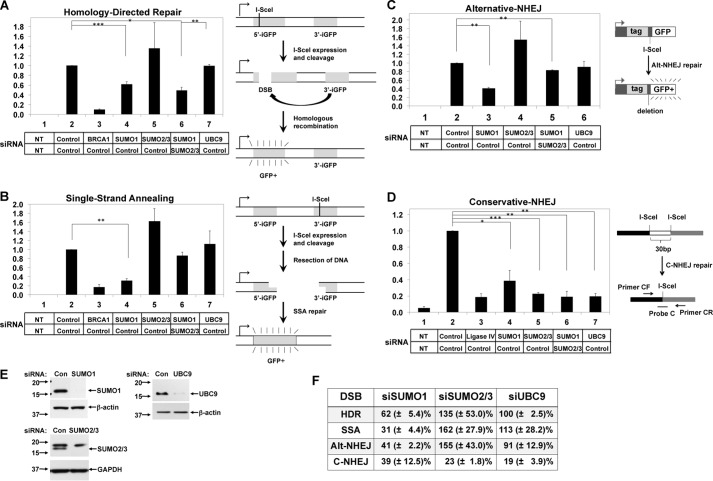
FIGURE 1.
SUMO isoforms function differently in DSB repair pathways. A–C, the recombination substrates are diagrammed on the right with details described previously (30–32). iGFP indicates inactive GFP gene. HeLa-derived cell lines for HDR (A), SSA (B), and Alt-NHEJ (C) were subjected to two rounds of siRNA transfection, as indicated, followed by transfection of the I-SceI expression plasmid to induce DSB. After 3 days, the percentages of GFP-positive cells were determined by flow cytometry. In each experiment, the percentage of GFP-positive cells from control siRNA transfections was set equal to 1, and the fraction of GFP-positive cells was determined relative to the control siRNA (Con) to measure HDR, SSA, and Alt-NHEJ, respectively. Results (mean ± S.E.) are from three independent experimental replicates. NT indicates no transfection of the I-SceI-expressing plasmid. D, the C-NHEJ repair substrate in the genome of 293 cells is diagrammed on the right as described previously (33). C-NHEJ assay was done by transfecting cells with the indicated siRNAs as in panel A. After 3 days, the repair efficiency was measured by quantitative real-time PCR on extracted genomic DNA, represented by the percentage on the y axis. In each experiment, the yield of repaired DNA was normalized relative to the value of the result from the control siRNA transfection. E, immunoblots show the depletion of indicated protein by RNAi interference in HeLa cells. Upon siSUMO2/3 transfection, the bottom band (∼15 kDa) of the doublet was depleted. GAPDH and β-actin were used as loading controls. The positions of the molecular mass markers in kDa are indicated at the left. F, results (mean ± S.E.) from each functional DSB repair assay were summarized for the indicated siRNA transfection.