FIGURE 1.
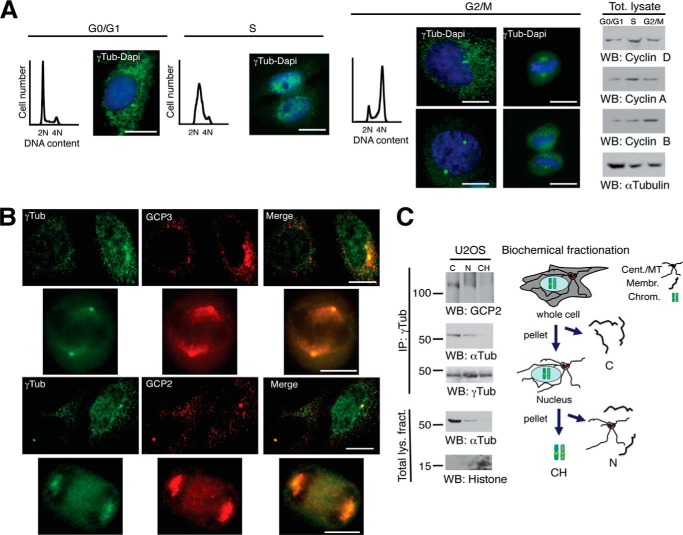
Nuclear γ-tubulin is not associated with GCP2 or GCP3. A, cells were synchronized in G0/G1 by keeping cell confluence during 48 h (G0/G1) or in early S phase by double thymidine block and released for 5 h (S) or 9 h (G2/M). Cell cycle progression was monitored by determining the DNA content of cells by flow cytometry (graphs) and by analyzing the protein levels of the G1 marker cyclin D, the S-phase marker cyclin A, and the G2/M marker cyclin B in cell lysates (Tot. lysate) by Western blotting (right panels). A and B, localization of endogenous γ-tubulin was examined by immunofluorescence staining with anti-γ-tubulin (green; rabbit) and anti-GCP3 (red; mouse) or anti-γ-tubulin (green; mouse) and anti-GCP2 (red; rabbit) antibodies, and nuclei were detected using DAPI (blue) in U2OS cells (n = 3–5). Scale bars, 10 μm. C, cells (20 × 106) were biochemically divided into the following cell fractions: cytosolic (C), nuclear membrane (N), and chromatin (CH). Each fraction was subjected to immunoprecipitations (IP) with an anti-γ-tubulin antibody and developed by Western blotting (WB) with an anti-GCP2 antibody (top), and then reprobed with α-tubulin (αTub) and γ-tubulin (γTub). Aliquots of the lysates used in the immunoprecipitations were run as loading controls (Total lys. fract.) and analyzed by Western blotting (n = 3). Models depict the cellular distribution of centrosomes/microtubules (Cent/MT), membranes (Membr.), and chromosomal (Chrom.) elements in the analyzed biochemical fractions.