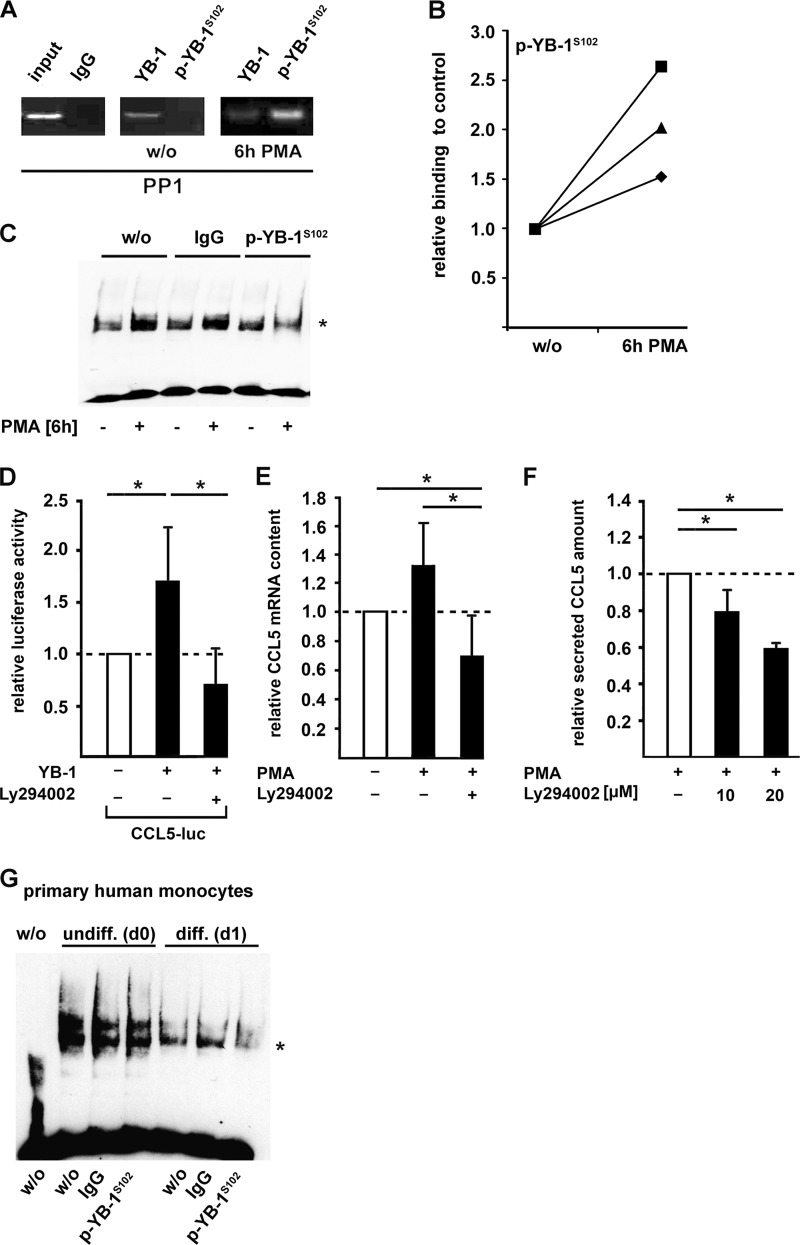
FIGURE 2.
Phosphorylation of YB-1 at Ser-102 increases its binding affinity to CCL5 promoter region and CCL5 expression in monocytes (THP-1 cells). A, ChIP assays were performed in THP-1 cells incubated with PMA for 6 h using p-YB-1S102-specific antibody or unspecific IgG (negative control). The amount of included DNA was tested without preceding immunoprecipitation (input). B, protein-DNA binding from A was quantified and confirmed by qRT-PCR in three independent experiments. C, EMSA analyses were performed with the antisense Y-box region within the human CCL5 promoter including nuclear protein extracts from THP-1 cells previously incubated with PMA (100 nm, 6 h) or left untreated. Participation of p-YB-1S102 in the complex formation was confirmed by supershift analyses (*) using a p-YB-1S102-specific or nonspecific IgG-antibody as control. D, CCL5 promoter activity was determined in THP-1 cells transfected with a plasmid harboring the proximal 1014 bp of the 5′ regulatory sequence covalently coupled to luciferase reporter gene and with a plasmid encoding for empty vector, wt-YB-1, respectively. Prior to PMA stimulation (100 nm, 6 h), THP-1 cells were preincubated with Akt inhibitor Ly294002 (10 μm) or vehicle for 2 h before CCL5 promoter activation. CCL5 mRNA (E) and secretion (F) was assessed by TaqMan and ELISA technology, respectively. Values were normalized to control transfection. Experiments were performed in at least three independent experiments, each performed in triplicate. Data are expressed as mean values ± S.D. G, EMSA analyses were performed with the antisense Y-box region within the human CCL5 promoter including nuclear protein extracts from human primary monocytes previously incubated with human serum for 24 h or left untreated. Participation of p-YB-1S102 in the complex formation was confirmed by supershift analyses (*) using a p-YB-1S102-specific or nonspecific IgG antibody as control.