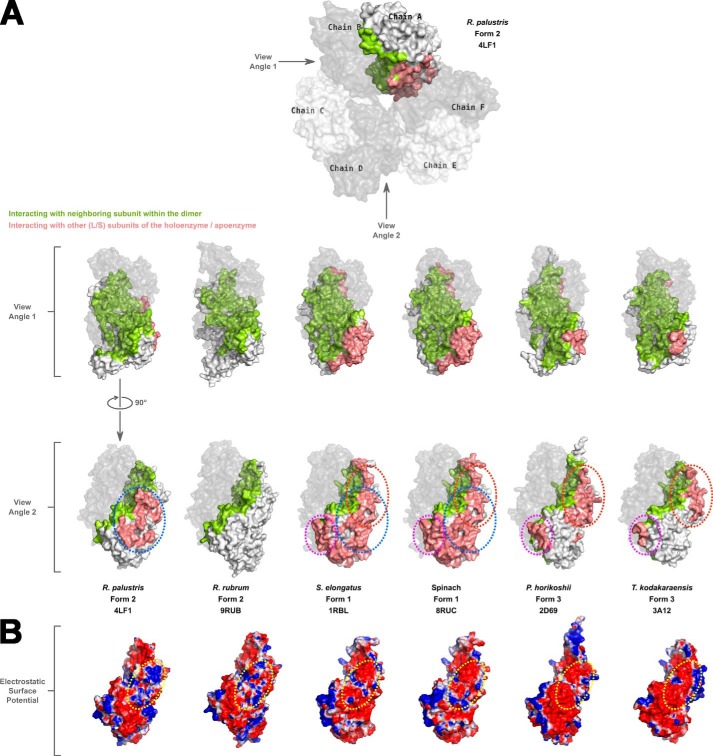
FIGURE 4.
A, a space-filling surface comparison of the large subunit dimers in different Rubiscos at two different angles 90° apart relative to the vertical axis. In each structure, one of the chains forming a dimer is shown in gray with reduced transparency. Residues in the other subunit forming the dimer that are involved in interactions with the dimer partner are colored green. Salmon-colored residues are involved in interactions with neighboring subunit(s) other than its dimer partner. White-colored surfaces are solvent-exposed. Areas circled with dotted lines indicate the common regions utilized by different Rubiscos for interactions with neighboring subunits other than its dimer partner. The color scheme and dotted lines are consistent with what is shown in Fig. 3. B, electrostatic surface representation of large subunit monomers at view angle 2 showing the differences in the interdimer interaction surfaces among the different Rubiscos. For R. rubrum Rubisco, which forms only a dimer, the analogous region is shown for comparison. The dotted yellow circle highlights the analogous regions in the different enzymes with the most variable electrostatic surfaces.