FIGURE 4.
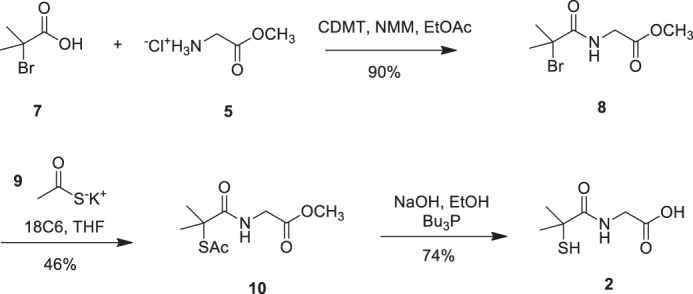
The route utilized to obtain dimethyl tiopronin analog 2 is shown. Commencing from commercially available 2-bromo-2-methylpropionic acid (7), 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT)-mediated coupling with glycine methyl ester hydrochloride (5) afforded bromoamide 8 in 90% yield, which was then reacted with thioacetate salt (9) to give (10) in 46% yield. Compound (10) was subjected to saponification with 2 n NaOH in the presence of tributylphosphine to afford dimethyl tiopronin analog (2) in 74% yield.
