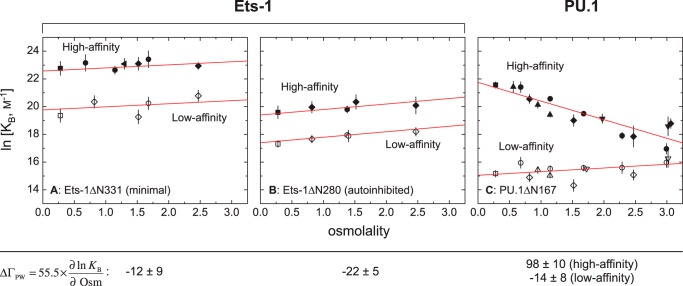
FIGURE 2.
Site-specific ETS·DNA complexes are osmotically sensitive in a sequence-dependent manner for PU.1, but not Ets-1. The role of preferential hydration in sequence-specific binding to high (solid symbols) and low affinity (open symbols) is determined by osmotic stress. Affinity is expressed as the binding constant, KB in units of m−1. A, the minimal ETS domain of Ets-1 (Ets-1ΔN331). B, the autoinhibited ETS domain of Ets-1 (Ets-1ΔN280). C, the ETS domain of PU.1 (PU.1ΔN167). Negative controls (without added osmolyte) are shown as squares. The osmolytes used are: betaine (diamonds), triethylene glycol (circles), glycerol (hexagons), nicotinamide (up triangles), sucrose (down triangles), and maltose (left triangles). The data for high and low affinity binding by Ets-1 are fitted to a common slope for each construct. The data for PU.1 are from experiments performed by Poon (18) under identical solution conditions.