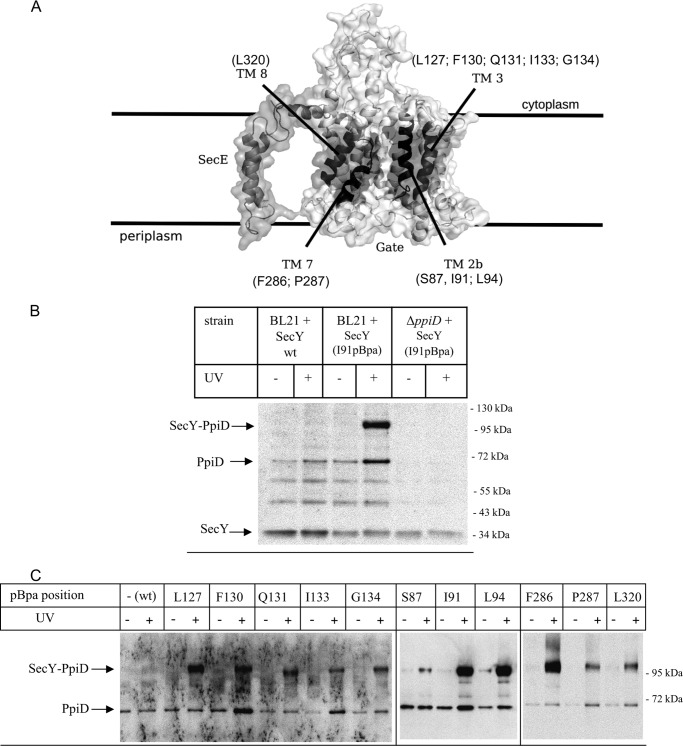
FIGURE 2.
The chaperone PpiD is located at the lateral gate of SecY. A, cryo-EM structure of the E. coli SecYEG complex based on the study by Frauenfeld et al. (15) (Protein Data Bank codes 3J00 and 3J01) with the lateral gate consisting of the four transmembrane domains highlighted in dark. pBpa was incorporated at the indicated residues. B, E. coli BL21 expressing either wild type SecY or SecY(I91pBpa) were treated as described under “Experimental Procedures.” As control, SecY(I91pBpa) was expressed in a ΔppiD strain. After SecY purification, samples were probed with antibodies against PpiD. Indicated are the SecY-PpiD cross-link, PpiD that co-purified with SecY, and the SecY band itself that cross-reacted with the PpiD antibody. The two bands at approximately 50 and 60 kDa probably correspond to proteolysis products of PpiD, because they were not detected in the ΔppiD strain, but this was not further analyzed. C, E. coli cells expressing either wild type SecY or SecY containing pBpa at the indicated positions were kept in the dark or were UV-exposed. Subsequently, SecY was purified and after Western transfer decorated with α-PpiD antibodies.