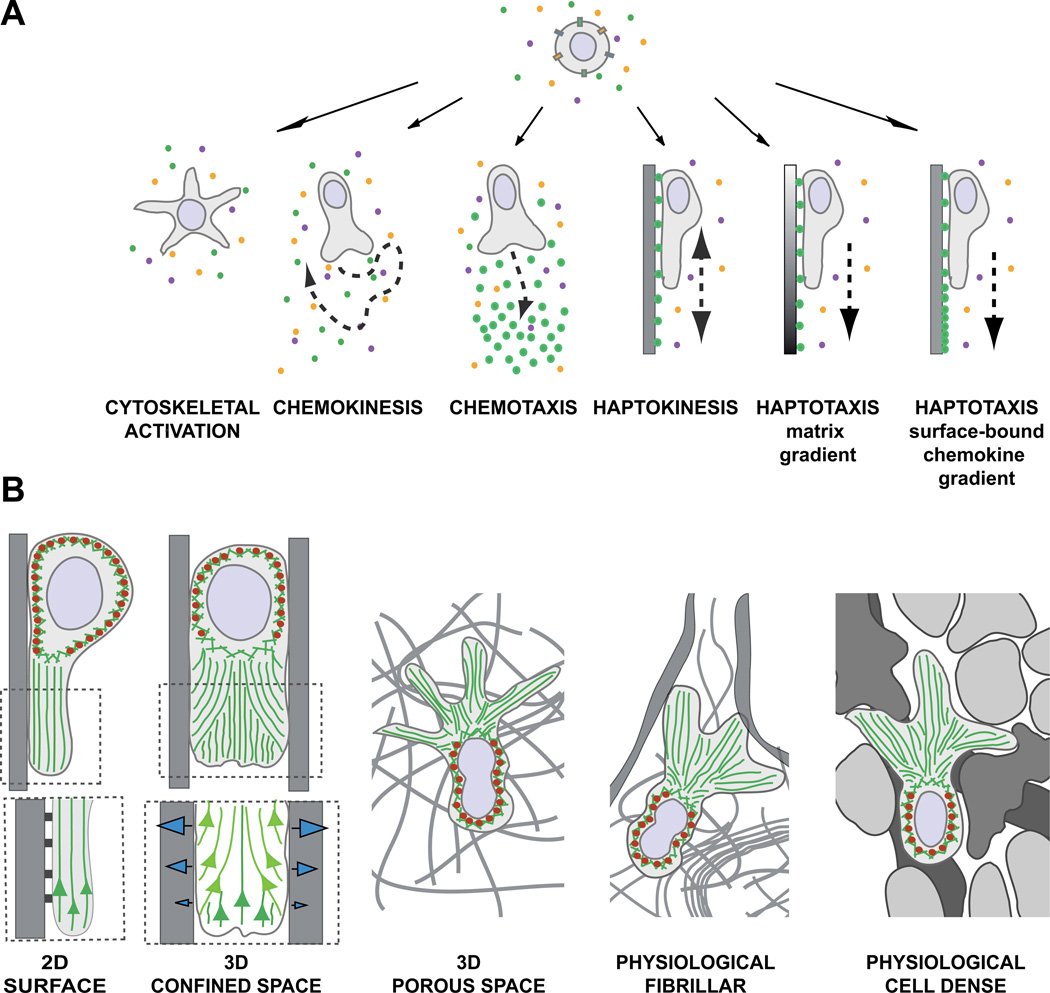
Fig. 1.
(A) Environmental signals induce shape changes, motility patterns, and migration modes. In physiological environments, leukocytes perceive multiple signals and integrate them into intracellular signaling cascades to induce shape changes, cell polarization, or cell migration. In a homogeneous field of soluble ligands, leukocytes can (1) increase cytoskeletal activity, leading to morphological shape changes without cell movement or (2) induce self-polarization as prerequisite for nondirected cell migration (chemokinesis). (3) Soluble gradients of external ligands can polarize cells along the gradient and stimulate directed migration (chemotaxis). (4) Upon perceiving homogeneous surface-bound chemokines, leukocytes can increase cell adhesion, self-polarize, and confine their migration in a nondirected manner along the surface (haptokinesis). For inherently adhesive immune cell types, haptokinesis might not require the presence of an activating chemokine (not shown). Leukocytes can migrate by two forms of haptotaxis: (5) along a gradient of extracellular matrix and cellular adhesion sites and (6) along a gradient of substrate-bound chemoattractants. (B) The environmental geometry supports different leukocyte migration strategies. (1) Movement along 2D surfaces requires actin polymerization to push the leading edge membrane forward and surface anchorage to the substrate. Myosin II (red ellipses)-based contractions behind the leading edge detach very adhesive cells from the substrate and are not necessarily required when cells migrate on low adhesive substrates. (Insert below) Integrin-mediated adhesions not only confine migration to the surface, but also inhibit the retrograde actin flow (green), converting actin polymerization into forward protrusion at the leading edge. (2) In confined environments, the leukocyte body can exert lateral pushing forces on the substrate, which is not possible on 2D surfaces. Intracellular pressure gradients increase friction against the walls and potentially compensate for adhesion-dependent anchorage. Friction against the walls can be generated by actin polymerization or increase in hydrostatic pressure by actomyosin contractions. (Insert below) Model of leading edge actin flow during adhesive migration in microchannels (adapted from [20]): Two F-actin networks form at the leading edge and interact with each other. The “free” network (dark green) polymerizes from the membrane at the cell front. The “adherent” network (light green) polymerizes perpendicular to the walls and compresses the retrograde flow of the “free” network, converting new polymerization against the membrane and enabling forward protrusions (see text for molecular details). (3) In 3D porous environments, leukocytes can potentially exert the same lateral forces as in confined spaces, but require additional mechanisms to navigate through meshes, which are not necessary when moving in the open space of a microchannel. Depending on the leukocyte subtype, multiple frontal protrusions require coordinated cell shape changes for efficient movement. (4, 5) In physiological tissues, leukocytes navigate in a very heterogeneous environment with confined spaces, fibrillar 3D meshes, and 2D surfaces in close proximity, most likely adapting to the tissue geometry by constant switching of migration modes. Fibrillar (4) and cell-rich (5) tissues differ in their structure, composition, and texture, potentially favoring distinct migration strategies.