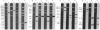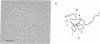Abstract
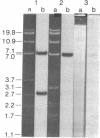
Hybrid phages that contain chicken lysozyme gene sequences have been isolated from a chicken DNA library. Two overlapping clones covering a region of 22 kilobase pairs around this gene have been studied by restriction mapping. Southern hybridization, and electron microscopic analysis of hybrids between lysozyme mRNA and the cloned cellular DNA. Three intervening sequences interrupt the lysozyme structural gene. The cellular gene is at least 3.9 kilobases long, about 6 times the length of the structural gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci P., Royal A., Cami B., Perrin F., Krust A., Garapin A., Kourilsky P. Isolation of the lysozyme gene of chicken. Nucleic Acids Res. 1979 Jun 25;6(8):2667–2681. doi: 10.1093/nar/6.8.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Stein J. P., Lai E. C., Woo S. L., Dugaiczyk A., Mace M. L., Means A. R., O'Malley B. W. The chick ovomucoid gene contains at least six intervening sequences. Nature. 1979 Mar 22;278(5702):323–327. doi: 10.1038/278323a0. [DOI] [PubMed] [Google Scholar]
- Chan L., Means A. R., O'Malley B. W. Rates of induction of specific translatable messenger RNAs for ovalbumin and avidin by steroid hormones. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1870–1874. doi: 10.1073/pnas.70.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. F. Estrogen withdrawal in chick oviduct. Selective loss of high abundance classes of polyadenylated messenger RNA. Biochemistry. 1977 Jul 26;16(15):3433–3443. doi: 10.1021/bi00634a022. [DOI] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Emtage J. S. Quantitation of ovalbumin mRNA in hen and chick oviduct by hybridization to complementary DNA. Accumulation of specific mRNA in response to estradiol. Eur J Biochem. 1974 Nov 1;49(1):225–236. doi: 10.1111/j.1432-1033.1974.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Woo S. L., Colbert D. A., Lai E. C., Mace M. L., Jr, O'Malley B. W. The ovalbumin gene: cloning and molecular organization of the entire natural gene. Proc Natl Acad Sci U S A. 1979 May;76(5):2253–2257. doi: 10.1073/pnas.76.5.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Jeep S., Wurtz T., Nguyen-Huu M. C., Giesecke K., Schütz G. Control of cellular content of chicken egg white protein specific RNA during estrogen administration and withdrawal. Biochemistry. 1979 Feb 20;18(4):616–624. doi: 10.1021/bi00571a011. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Nguyen-Huu M. C., Schütz G. mRNA complexity and egg white protein mRNA content in mature and hormone-withdrawn oviduct. Cell. 1977 Aug;11(4):923–932. doi: 10.1016/0092-8674(77)90303-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Nguyen-Huu M. C., Barrett K. J., Giesecke K., Wurtz T., Sippel A. E., Schütz G. Transcription of the chicken ovalbumin and conalbumin gene during early secondary induction with estrogens. Hoppe Seylers Z Physiol Chem. 1978 Oct;359(10):1307–1313. doi: 10.1515/bchm2.1978.359.2.1307. [DOI] [PubMed] [Google Scholar]
- Nguyen-Huu M. C., Sippel A. A., Hynes N. E., Groner B., Schütz G. Preferential transcription of the ovalbumin gene in isolated hen oviduct nuclei by RNA polymerase B. Proc Natl Acad Sci U S A. 1978 Feb;75(2):686–690. doi: 10.1073/pnas.75.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Huu M. C., Stratmann M., Groner B., Wurtz T., Land H., Giesecke K., Sippel A. E., Schütz G. Chicken lysozyme gene contains several intervening sequences. Proc Natl Acad Sci U S A. 1979 Jan;76(1):76–80. doi: 10.1073/pnas.76.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Carey N. H. Rapid inactivation of ovalbumin messenger ribonucleic acid after acute withdrawal of estrogen. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2357–2361. doi: 10.1073/pnas.71.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Palmiter R. D., Smith L. T. Synergistic effects of oesterogen and progesterone on ovomucoid and conalbumin mRNA synthesis in chick oviduct. Nat New Biol. 1973 Nov 21;246(151):74–76. doi: 10.1038/newbio246074a0. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Schimke R. T. Differential effects of estrogen and progesterone on ovalbumin mRNA utilization. J Biol Chem. 1978 Dec 25;253(24):8925–8934. [PubMed] [Google Scholar]
- Royal A., Garapin A., Cami B., Perrin F., Mandel J. L., LeMeur M., Brégégègre F., Gannon F., LePennec J. P., Chambon P. The ovalbumin gene region: common features in the organisation of three genes expressed in chicken oviduct under hormonal control. Nature. 1979 May 10;279(5709):125–132. doi: 10.1038/279125a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Schütz G., Nguyen-Huu M. C., Giesecke K., Hynes N. E., Groner B., Wurtz T., Sippel A. E. Hormonal control of egg white protein messenger RNA synthesis in the chicken oviduct. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):617–624. doi: 10.1101/sqb.1978.042.01.064. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Land H., Lindenmaier W., Nguyen-Huu M. C., Wurtz T., Timmis K. N., Giesecke K., Schütz G. Cloning of chicken lysozyme structural gene sequences synthesized in vitro. Nucleic Acids Res. 1978 Sep;5(9):3275–3294. doi: 10.1093/nar/5.9.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swaneck G. E., Nordstrom J. L., Kreuzaler F., Tsai M. J., O'Malley B. W. Effect of estrogen on gene expression in chicken oviduct: evidence for transcriptional control of ovalbumin gene. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1049–1053. doi: 10.1073/pnas.76.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]