Abstract
Aim:
The study investigated the direct effects of tramadol on the coagulation status of women with gynecologic malignancies in vitro.
Materials and Methods:
Citrated whole-blood samples from 21 patients with gynecologic tumors were spiked ex vivo with 2 or 6 μl/ml tramadol. Thrombelastography (TEG) analysis was performed using ROTEM® to assess clotting time (CT), clot formation time (CFT) and maximum clot formation (MCF).
Results:
In the INTEM assay, CT (P < 0.05) and CFT (P < 0.01) were significantly prolonged with tramadol at a 6 μl/ml concentration compared with baseline. There were no significant differences in MCF values between the baseline and the tramadol-treated samples (P > 0.05). Blood medicated with tramadol (6 μl/ml) clotted slowly (increased CT and CFT).
Conclusion:
The changes observed by TEG demonstrated that tramadol impairs hemostasis in a concentration-dependent manner in the whole blood of women with gynecologic malignancies in vitro.
KEY WORDS: Coagulation, malignancy, thromboelastography, tramadol
Introduction
Pain is a frequent symptom in clinical practice, particularly in patients with malignancy. There may be multiple sources of pain, including primary or metastatic origins. Clinicians must select an analgesic regimen that can control pain while minimizing side effects. Safety issues in analgesic prescription are of particular concern in relation to long-term treatment for the management of cancer-related pain.[1] However excruciating pain is not the only problem for cancer patients. The rising demand for pain management frequently coincides with increasing concerns about different system disorders related to the primary disease. Patients usually have to face hematological disorders related to the primary disease or chemotherapies. Changes in coagulation status and a tendency toward thromboembolic complications in patients with gynecologic cancer have been reported in many studies.[2,3,4]
Additionally, surgery is an important factor contributing to hypercoagulable states. In this context, any agent that aggravates a hypercoagulable state would be catastrophic, particularly in those patients undergoing surgery or in patients confined to bed in the terminal cancer period. However, invasive pain management techniques, such as epidurals and spinal catheterizations, are commonly used for opioid and local anesthetic administration either for postoperative analgesia or for intractable pain in terminally ill patients. Any concomitantly used agent that exerts hypocoagulable characteristics may lead to coagulation derangement and a subsequent risk of epidural hematoma. Therefore, it is of great importance for clinicians to understand the effect of analgesic agents on the coagulation system in cancer patients.
Tramadol is a widely used analgesic in cancer patients. This drug is a centrally acting atypical opioid with proven analgesic efficacy and tolerability as a single agent. This efficacy appears to result from multiple modes of action, including both opioid and monoaminergic components.[5] The commonly known adverse effects are nausea, dizziness, sedation, dry mouth, sweating and headache, all of which are generally mild. To our knowledge, there is no report on the direct effects of this agent on coagulation status. Therefore, the purpose of our study was to investigate the direct effects of tramadol on thrombelastography (TEG) parameters in women with gynecologic malignancies in vitro.
Materials and Methods
The Institutional Ethics Committee of our university approved this study on volunteers with gynecologic malignancy. Those with a history of hematological or coagulation disorders, taking anticoagulant therapy and with renal and liver dysfunctions were excluded. Written permissions were obtained from the patients themselves.
Blood was collected from 21 participants (11 had endometrial cancer and 10 cervical cancer). The first 2 ml blood was discarded to prevent tissue thromboplastin contamination before drawing samples for TEG analysis into 4.5 ml vacutainers (Becton Dickinson) containing 3.2% trisodium citrate with a citrate/blood ratio of 1:9. Two different doses of tramadol (2 μl/ml or 6 μl/ml) were spiked ex vivo to the citrated whole blood samples in 2 ml vials.[6] The coagulation of whole blood (baseline) and blood with different concentrations of tramadol was assessed using TEG.
A ROTEM® Coagulation Analyzer (Pentapharm, Munich, Germany) was utilized for TEG and the samples were analyzed within 30-90 min of blood collection by the same investigator. In total, 300 μl citrated blood was re-calcified with 20 μl 0.2 mol/l CaCl2 (star-TEM®; Pentapharm, Munich, Germany) after incubating the test solution at 37°C for 2 minutes. Both INTEM and EXTEM analyses were performed according to the standard procedure recommended by the manufacturer. In INTEM analysis, coagulation was activated by 20 μl contact activator (partial thromboplastin-phospholipid from rabbit brain extract and ellagic acid; in-TEM®; Pentapharm, Munich, Germany). In EXTEM analysis, coagulation was activated by 20 μl tissue factor (TF; tissue thromboplastin from rabbit brain extract; ex-TEM®; Pentapharm, Munich, Germany). The parameters obtained from ROTEM® analysis were clotting time (CT), reflecting the initiation of coagulation; clot formation time (CFT), reflecting the rate of clot formation once formation is initiated; and maximum clot firmness (MCF), representing the firmness of the clot. The method and the parameters of ROTEM® have been previously described in detail.[2]
Statistical analysis was performed using Statistical Package for the Social Sciences software (SSPS) IBM Statistics 20. Normally distributed continuous dependent variables were analyzed using two-way analysis of variance (ANOVA). Parametric Tukey's post-hoc multiple comparison tests were used to perform comparisons between different drug concentrations, and values are given as the mean ± standard deviation (SD). Non-normally distributed variables were analyzed using Friedman's two-way ANOVA. A nonparametric Tukey's post-hoc multiple comparison test was used to compare the different drug concentrations, and the data are presented as the median (25th to 75th percentile) and the mean ± SD. The P value less than 0.05 was considered statistically significant.
Results
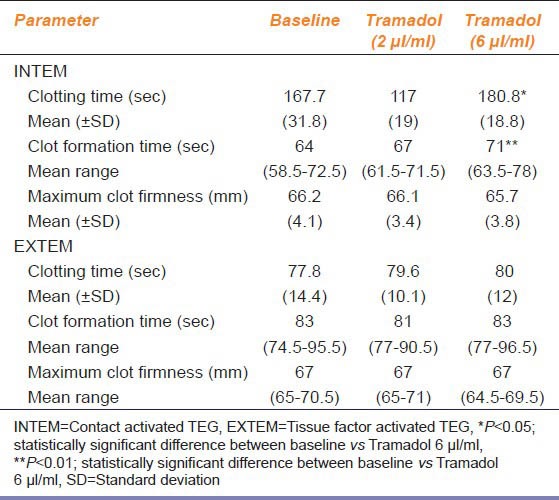
In total, 21 patients with gynecologic malignancies at similar stages were included in the study. ROTEM® parameters of three samples (whole blood, tramadol 2 μl/ml and tramadol 6 μl/ml) are presented in Table 1. In the INTEM assay, CT (P < 0.05) and CFT (P < 0.01) were significantly prolonged with the addition of tramadol at a 6 μl/ml concentration compared with the baseline values, whereas maximum clot firmness showed no significant difference between the baseline and the tramadol-treated samples. Blood medicated with high-dose tramadol (6 μl/ml) clotted slowly (increased CT and CFT). In the EXTEM assay, there was no significant difference in CT, CFT and MCF values between the baseline and the tramadol-treated samples.
Table 1.
Results of the ROTEM® parameters in whole blood without tramadol and with two different concentrations of tramadol
Discussion
The results of our study confirm that tramadol affects the coagulation system in vitro. The changes in coagulation observed using ROTEM® demonstrated that tramadol impairs hemostasis in a concentration-dependent manner in women with gynecologic malignancies.
Tramadol is an effective analgesic agent used for the management of moderate-to-severe pain, and this agent has continued to be the mainstay of the armamentarium against pain in cancer patients beyond other opioids. Many studies have described the safe use of tramadol in patients undergoing surgery in terms of hemodynamic and respiratory side effects. However, there has been no clinical or laboratory study on the direct effects of tramadol on coagulation status in humans. In a trial that compared tramadol hydrochloride and diclofenac sodium in terms of analgesic efficacy and hemorrhage following a tonsillectomy operation, two patients in the tramadol group (n = 24) and one patient in the diclofenac group (n = 25) were admitted to the hospital for post-tonsillectomy hemorrhage.[7] In another study, performed in adult patients undergoing tonsillectomy, it was reported that preoperatively administered tramadol and lornoxicam have a similar rate of side effects, including bleeding.[8] De Decker et al. described a tramadol overdose in a young man and reported pathologic changes such as diffuse hemorrhagic mucosa of the gastrointestinal tract and alveolar hemorrhages in the lung beyond evidence of multiple organ failure.[9] There are only two animal studies that have assessed the effect of tramadol on coagulation. A trial in rabbits demonstrated that tramadol and droperidol enhance the coagulation of plasma proteins and suppress the thrombocyte deaggregation process.[10] However, in another animal model, it was demonstrated that tramadol was not associated with significant changes in primary hemostasis.[11] These reports indicate that the effect of tramadol on coagulation status remains unclear and controversial.
There are several reasons why the effect of tramadol on coagulation status is thought to be important. First, tramadol displays anti-inflammatory effects,[5,12] the mechanism of which is unclear. It may be because of cyclooxygenase inhibition, which also exerts an anticoagulant effect. Second, tramadol and morphine exert similar action on α-2 adrenoreceptors.[13,14] Hsiao et al., mentioned that morphine potentiates agonist-induced platelet aggregation by the activation of α-2 adrenoreceptors and the mobilization of intracellular calcium.[13] Last, another mode of tramadol action is a local anesthetic effect.[5] Local anesthetics may induce changes in the membrane of platelets, inhibiting alpha-granule release, thromboxane A2 signaling and aggregation by blocking membrane ion channels.[15] Tramadol may also exert effects on membrane ion channels, similar to local anesthetics.[15] However, all of these mechanisms are assumptive and need further investigation. Our present data does not establish a clear mechanism of action for tramadol's effect on coagulation status.
In our study, we aimed to determine thrombolestographic changes to observe whether tramadol influences the coagulation status of patients with gynecologic malignancies. From our results, it is clear that increased tramadol concentrations affected the initiation and speed of CF, as reflected by the prolongation of CT and CFT values determined by the INTEM analysis. Tramadol seems to impair hemostasis and increasing the bleeding tendency by affecting the activity of coagulation factors rather than platelets and fibrinogen levels, as MCF values did not show a statistically significant difference recorded in the present study. The results of this study suggest that tramadol may be useful for patients who have a tendency toward a hypercoagulable status. A hypocoagulatory effect, defined by a reduction in final clot strength, may not necessarily be a negative outcome in the case of a hypercoagulable status and a tendency toward thromboembolic complications. Nonetheless, the clinical significance of these findings needs further investigation.
The clinical relevance of this in vitro study has certain limitations. We only studied thrombelastographic variables in cancer patients who were inclined towards a hypercoagulable status. Subsequent studies should investigate the effect of tramadol in a different group of patients. Another limitation may be the dose and incubation time of tramadol. The inhibitory effect of tramadol may increase at different concentrations and longer incubation times because tramadol may have prolonged contact with the blood under clinical conditions of intravenous, patient-controlled analgesia. In conclusion, the results suggest that tramadol causes hypocoagulable changes in the thrombelastographic profile of gynecologic cancer patients in vitro. The results of our study need to be confirmed by follow-up clinical studies.
Acknowledgments
We thank Fezan Sahin Mutlu, PhD, for the statistical analysis. We also thank Esin Kus for laboratory assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Langford RM. Pain management today-what have we learned? Clin Rheumatol. 2006;(25 Suppl):S2–8. doi: 10.1007/s10067-006-0311-5. [DOI] [PubMed] [Google Scholar]
- 2.Akay OM, Ustuner Z, Canturk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med Oncol. 2008;26:358–64. doi: 10.1007/s12032-008-9129-0. [DOI] [PubMed] [Google Scholar]
- 3.Wehrum MJ, Hines JF, Hayes EB, Kost ER, Hall KL, Paidas MJ. Comparative assessment of hypercoagulability in women with and without gynecologic malignancies using the thromboelastograph coagulation analyzer. Blood Coagul Fibrinolysis. 2010;21:140–3. doi: 10.1097/MBC.0b013e3283358179. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Lee A, Critchley LA, White PF. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108:734–42. doi: 10.1213/ane.0b013e31818f8907. [DOI] [PubMed] [Google Scholar]
- 5.Leppert W. Tramadol as an analgesic for mild to moderate cancer pain. Pharmacol Rep. 2009;61:978–92. doi: 10.1016/s1734-1140(09)70159-8. [DOI] [PubMed] [Google Scholar]
- 6.Beilin B, Grinevich G, Yardeni IZ, Bessler H. Tramadol does not impair the phagocytic capacity of human peripheral blood cells. Can J Anaesth. 2005;52:1035–9. doi: 10.1007/BF03021601. [DOI] [PubMed] [Google Scholar]
- 7.Courtney MJ, Cabraal D. Tramadol vs. diclofenac for posttonsillectomy analgesia. Arch Otolaryngol Head Neck Surg. 2001;127:385–8. doi: 10.1001/archotol.127.4.385. [DOI] [PubMed] [Google Scholar]
- 8.Isik B, Arslan M, Ozsoylar O, Akcabay M. Effects of preoperative lornoxicam versus tramadol on postoperative pain and adverse effects in adult tonsillectomy patients. Agri. 2009;21:113–20. [PubMed] [Google Scholar]
- 9.De Decker K, Cordonnier J, Jacobs W, Coucke V, Schepens P, Jorens PG. Fatal intoxication due to tramadol alone: Case report and review of the literature. Forensic Sci Int. 2008;175:79–82. doi: 10.1016/j.forsciint.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Bol’shakov VV, Sapozhkov AV, Denisova SV. Effect of droperidol and tramadol combination on the hemostasis in rabbits. Eksp Klin Farmakol. 2002;65:50–2. [PubMed] [Google Scholar]
- 11.Brondani JT, Luna SP, Marcello GC, Padovani CR. Perioperative administration of vedaprofen, tramadol or their combination does not interfere with platelet aggregation, bleeding time and biochemical variables in cats. J Feline Med Surg. 2009;11:503–9. doi: 10.1016/j.jfms.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buccellati C, Sala A, Ballerio R, Bianchib M. Tramadol anti-inflammatory activity is not related to a direct inhibitory action on prostaglandin endoperoxide synthases. Eur J Pain. 2000;4:413–5. doi: 10.1053/eujp.2000.0208. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao G, Shen MY, Fang CL, Chou DS, Lin CH, Chen TF, et al. Morphine-potentiated platelet aggregation in in vitro and platelet plug formation in in vivo experiments. J Biomed Sci. 2003;10:292–301. doi: 10.1007/BF02256448. [DOI] [PubMed] [Google Scholar]
- 14.Höcker J, Weber B, Tonner PH, Scholz J, Brand PA, Ohnesorge H, et al. Meperidine, remifentanil and tramadol but not sufentanil interact with alpha (2)-adrenoceptors in alpha (2A)-, alpha (2B)- and alpha (2C)- adrenoceptor knock out mice brain. Eur J Pharmacol. 2008;17(582):70–7. doi: 10.1016/j.ejphar.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Mert T, Gunes Y, Gunay I. Local analgesic efficacy of tramadol following intraplantar injection. Eur J Pharmacol. 2007;558:68–72. doi: 10.1016/j.ejphar.2006.11.055. [DOI] [PubMed] [Google Scholar]


