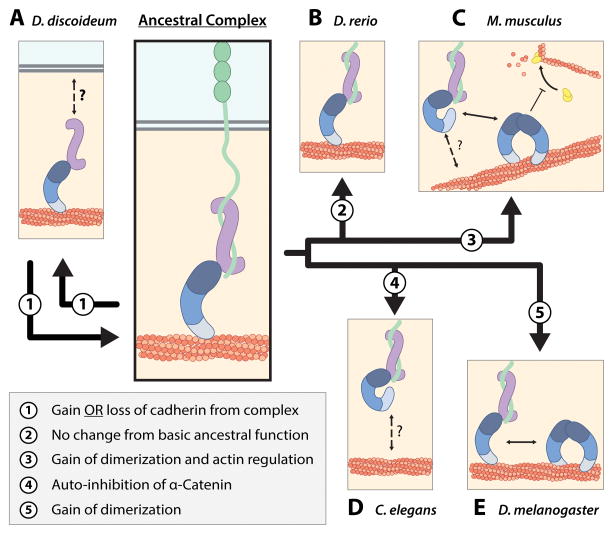
Figure 3. Functional Divergence of Cadherin-Catenin Complex.
(A) We propose that ancestral cadherin-catenin complex interactions consisted of monomeric actin-binding α-catenin that coupled the classical cadherin-β-catenin complex to the cortical actin network (center panel). Divergence of the complex in each species from the ancestral complex is depicted in the box on bottom left. D. discoideum α-catenin is an monomer that forms a heterodimer complex with Aardvark (β-catenin homolog), and localizes to cell-cell contacts and the cortical actin cytoskeleton. Dd α-catenin bundles actin, but the mechanism by which the Dd α-catenin-Aardvark complex associates with the cell membrane is not known. D. discoideum does not possess cadherin homologs and the adhesion proteins to which the heterodimer complex is attached at cell-cell contacts have not been identified.
(B) D. rerio αE-catenin is a monomer that is not auto-inhibited, and can simultaneously bind β-catenin and F-actin, but does not regulate actin dynamics via Arp2/3 inhibition.
(C) The mammalian cadherin-catenin complex possesses divergent regulatory properties from the ancestral complex. Monomeric αE-catenin associates with the E-cadherin-β-catenin complex, but is allosterically regulated and binds actin weakly. The mechanism by which the cadherin-catenin complex is linked to the cortical actin cytoskeleton is poorly understood. Homodimerization of αE-catenin potentiates bundling of actin and inhibition of Arp2/3 complex-mediated actin polymerization.
(D) C. elegans HMP-1 forms a ternary complex with HMR-1 (classical cadherin homolog) and HMP-2 (β-catenin homolog), but is autoinhibited and cannot bind F-actin in vitro. The mechanism by which actin binding is activated in vivo is not known.
(E) D. melanogaster α-catenin exists as monomer and homodimer species, both of which bind actin. D. melanogaster α-catenin forms a ternary complex with DE-cadherin and Armadillo.