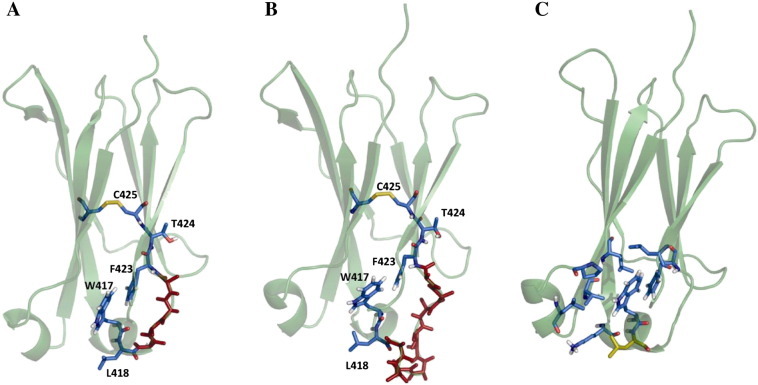
Fig. 1.
Graphical representation of IgG1-CH3-Fc. Residues of interest are shown in blue, sites of randomization and insertion in red (A and B). Most prevalent structures of two systems according to cluster analyses are shown. (A) The variant stem(0), bearing the two stabilizing mutations Q418L and S424T. Residues 419–422 were replaced by alanines. (B) The variant stem(5), differing from stem(0) between residues 419 and 422, where 5 additional alanines were inserted. (C) The variant Q418L/S424T. Amino acids contributing to a hydrophobic cluster on the C-terminal part of the CH3 domain are highlighted in blue. The mutation Q418L is shown in yellow.