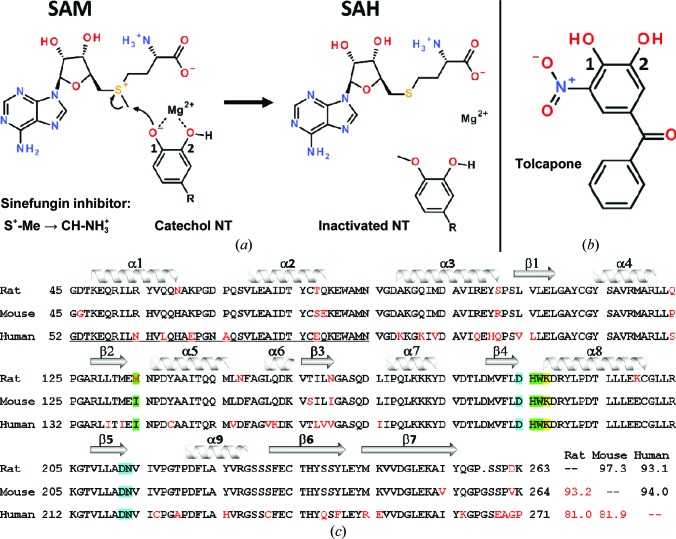
Figure 1.
Sequence–catalysis relationship of COMT. (a) Scheme of the catalysed reaction. A catechol NT is bound as a bidentate ligand to an Mg2+ ion and deprotonated to form a nucleophile that attacks the activated methyl group of SAM. The methylated NT is not able to complex Mg2+ and dissociates. The natural product and antibiotic sinefungin is an isoster of SAM, thus acting as an inhibitor of many SAM-dependent enzymes [e.g. structures (7) and (8)]. (b) Structure of tolcapone, an acidic catechol and COMT inhibitor. The modified and unmodified hydroxyl groups of the catechol are indicated by the numbers 1 and 2, respectively. (c) Sequence alignment of the catalytic domains of rat, mouse and human COMT. The N-terminal transmembrane sequences were ignored for the alignment. Sequence numbering for human COMT is shifted by seven with respect to the rodent enzymes. Secondary-structure elements are drawn at the top of the alignment. At the end of the alignment, red and black numbers are the percentage identity and similarity, respectively, between the sequence pairings. Red letters denote differences in at least one of the three sequences. Residues in the adenine and Mg2+-binding sites are highlighted with green and cyan backgrounds, respectively. The catalytic lysine residue has a yellow background.