Figure 2.
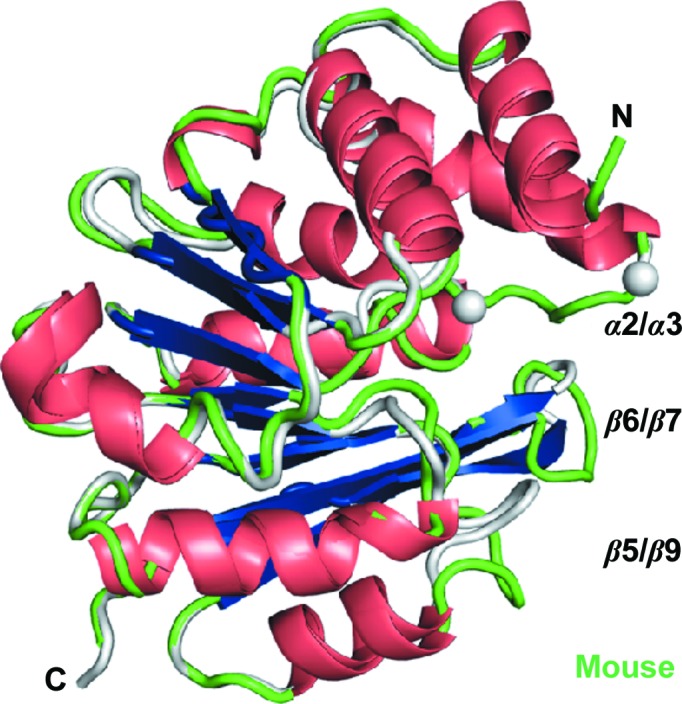
Novel human and mouse apo COMT crystal structures. The ribbon diagrams of human apo COMT (1) and mouse apo COMT (4) show a central seven-stranded β-sheet that is flanked by α-helices. The termini are marked, and three loop regions (grey for human COMT and green for mouse COMT) that are important for catalysis and for domain swapping are labelled α2/α3, β5/α9 and β6/β7. α2/α3 is disordered in this apo form of human COMT (indicated by grey spheres) but is ordered in mouse COMT.
