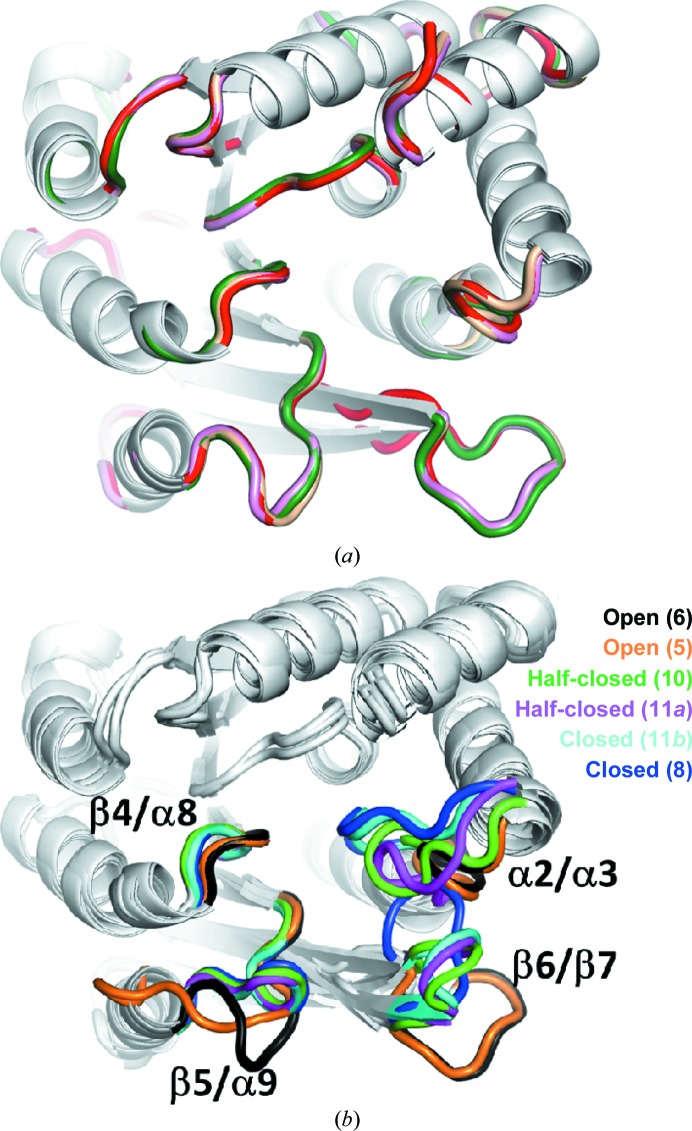
Figure 4.
Comparison of rodent apo COMT structures and overall conformational changes of rat COMT upon substrate binding. (a) Apo COMT structures from mouse (4) and rat (5, 6) adopt the same conformation. Mouse COMT is marked with green loops. The rat structure (5) contains four molecules in the asymmetric unit, two of which (wheat and pink) have the same conformation as mouse COMT (4). Another rat structure (6) in a different crystal form also shows the same conformation (red). The view is rotated 90° clockwise about the y axis with respect to Fig. 2 ▶. (b) Loop closure upon substrate binding to rat COMT. No ligands are shown for clarity and the loops that move most are coloured the same for each structure. Structure (6) (black) adopts the most open conformation, followed by (5), (10), the two conformations of (11) and structure (8), which are coloured orange, green, magenta, cyan and blue, respectively. Structure (11) contains two molecules per asymmetric unit that differ in their active-site loop conformations. Structures (7) and (9) have the same conformation as (10) (only the latter is shown)