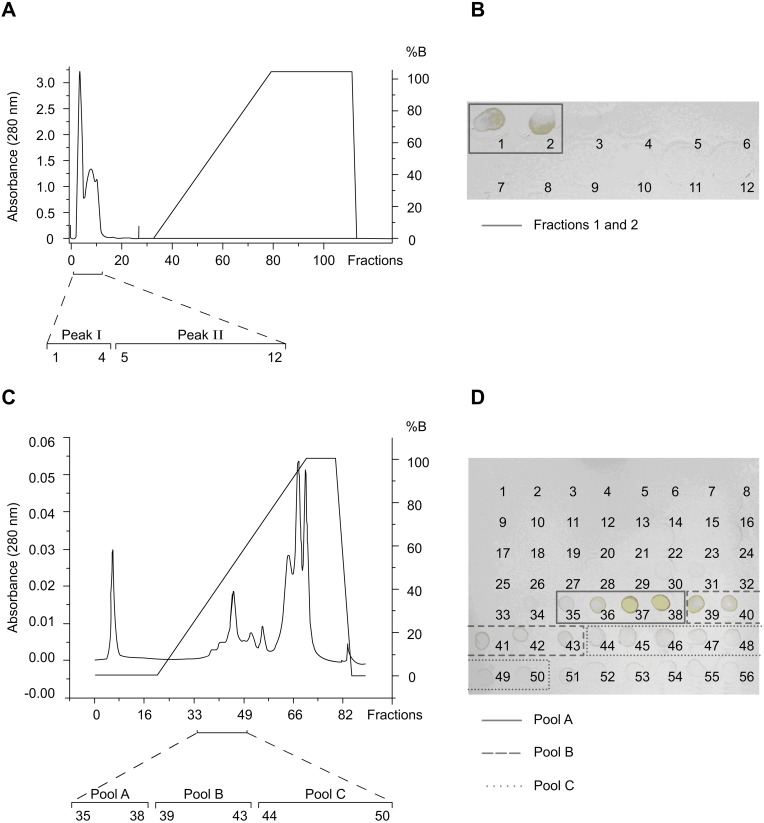
Figure 2. Partial purification of the mouse epididymal protein detected with mAb TRA 54.
A) Fractionation of a homogenate of epididymal caput tissue on a column of Sepharose-concanavalin A (Con-A). After washing the column with 0.1 M sodium acetate, pH 6.0, containing 1 M NaCl, 1 mM MgCl2 and 1 mM CaCl2, the column was eluted with a linear gradient (0–0.5 M) of glucose in this same buffer. Note that two peaks (I and II) eluted during column washing and no proteins were eluted with the glucose gradient. B) A dot blot of the fractions corresponding to peaks I and II showed that only fractions 1 and 2 of peak I reacted with the mAb TRA 54. These fractions were combined and purified further by anion exchange chromatography (HiTrap Q-Sepharose high performance column). C) Elution profile of immunoreactive fractions 1 and 2 (from peak I of the Sepharose-Con A step) after ion exchange chromatography. Bound proteins were eluted with a linear gradient of NaCl (0–1 M) in 50 mM Tris-HCl, pH 7.4. D) A dot blot identified several fractions that reacted with mAb TRA 54. The positive fractions were combined to form three pools (pool A: fractions 35–38; pool B: fractions 39–43; pool C: fractions 44–50) that were subsequently analyzed by electrophoresis and immunoblotting.