Figure 3. Expression and purification of GST-SID fusion protein.
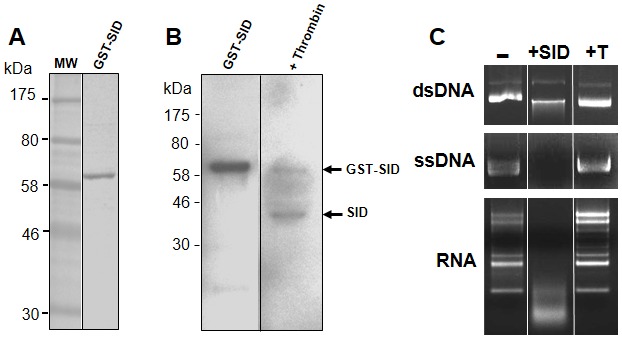
(A) A GST-SID fusion protein was expressed in bacteria and partially purified with glutathione beads. The GST-SID protein was separated as in Figure 1, and the eluted fraction was found to have few contaminants by protein staining (Coomassie R-250 blue). (B) Thrombin cleavage of the GST-SID fusion protein resulted in the release of the SID protein (arrow) as detected with anti-His antibodies (on the carboxyl-terminus of SID). (C) The thrombin-cleaved recombinant SID protein was examined for nuclease activity on DNA and RNA substrates in the presence of Cu++ (+SID) or in the presence of thrombin alone (+T). All reactions were carried as in Figure 1.
