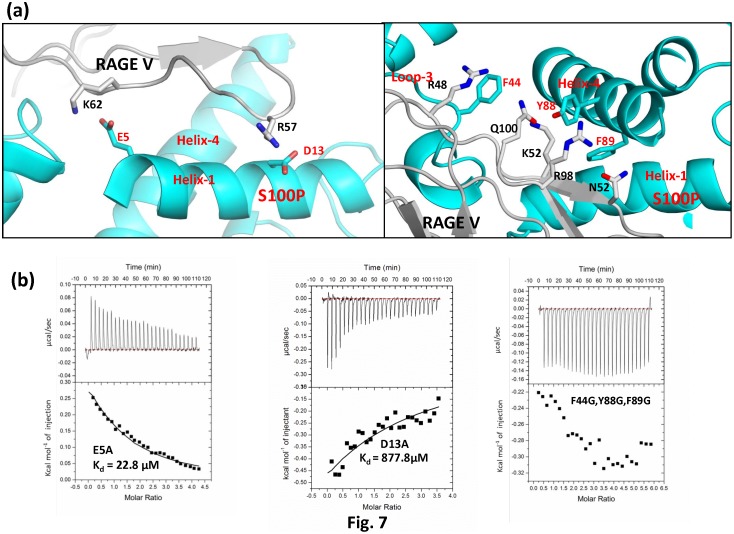
Figure 7. ITC titrations of the RAGE V domain with S100P mutants.
(a) Ribbon representation of the model of the S100P-RAGE V domain complex with the mutated polar charged residues and hydrophobic residues of S100P shown as sticks. Helix-1, Helix-4 and loop-3 of S100P are colored cyan, and RAGE V is colored gray with the residues that interact with the mutated S100P residues shown as sticks. (b) Binding properties of S100P E5A, S100P D13A, and S100P F44G/Y88G/F89G with RAGE V domain. The upper panels represent the raw data and whereas the bottom panels are the integrated plot of the amount of heat liberated per injection as a function of the molar ratio of the S100P mutants to RAGE V domain. The dissociation constants (Kd) for the RAGE V domain-S100P E5A interaction and the RAGE V domain-S100P D13A interaction were determined to be 23.6 µM and 877.8 µM, respectively. No binding of S100P F44G/Y88G/F89G to the RAGE V domain was detected.