Two splicing factors of the multipartite mitochondrial nad5 transcript are pentatricopeptide repeat proteins.
Abstract
Pentatricopeptide repeat proteins constitute a large family of RNA-binding proteins in higher plants (around 450 genes in Arabidopsis [Arabidopsis thaliana]), mostly targeted to chloroplasts and mitochondria. Many of them are involved in organelle posttranscriptional processes, in a very specific manner. Splicing is necessary to remove the group II introns, which interrupt the coding sequences of several genes encoding components of the mitochondrial respiratory chain. The nad5 gene is fragmented in five exons, belonging to three distinct transcription units. Its maturation requires two cis- and two trans-splicing events. These steps need to be performed in a very precise order to generate a functional transcript. Here, we characterize two pentatricopeptide repeat proteins, ORGANELLE TRANSCRIPT PROCESSING439 and TANG2, and show that they are involved in the removal of nad5 introns 2 and 3, respectively. To our knowledge, they are the first two specific nad5 splicing factors found in plants so far.
Chloroplasts and mitochondria are the organelles that supply the eukaryotic cell with energy via ATP synthesis. They derive from cyanobacteria and α-proteobacteria, respectively, via two independent endosymbiosis events (Andersson et al., 2003; Raven and Allen, 2003). Massive gene transfers between the organelles and the nucleus have occurred during the last billion years (Martin and Herrmann, 1998), leading to a reduction of organelle genome content. Many of the remaining organelle genes have acquired or retained introns, which derive from bacterial self-splicing group II intron ribozymes, but have lost their ability to self-splice. Expression of these organelle genes requires the participation of numerous nuclearly encoded factors that perform the posttranscriptional steps (editing, intron splicing, protection of the 3′ and 5′ ends, and stability) necessary for the production of translatable transcripts. Several families of such factors have been described, containing various RNA-binding domains such as CHLOROPLAST RNA SPLICING AND RIBOSOME MATURATION (CRM; Asakura and Barkan, 2006; Zmudjak et al., 2013), PLANT ORGANELLAR RNA RECOGNITION (Kroeger et al., 2009; Colas des Francs-Small et al., 2012), REGULATOR OF CHROMOSOME CONDENSATION (RCC; Kühn et al., 2011), MULTIPLE ORGANELLAR RNA EDITING FACTOR (Takenaka et al., 2012), and RNA EDITING-INTERACTING PROTEIN (Bentolila et al., 2012), but the pentatricopeptide repeat (PPR) protein family is by far the biggest, with around 450 genes found in Arabidopsis (Arabidopsis thaliana), where it was first described (Barkan and Small, 2014). These proteins are widely distributed among eukaryotes but particularly abundant in land plants. They are characterized by tandem repeats of a 35-amino acid motif (Small and Peeters, 2000) and classified according to the type of motifs they bear: members of the P subfamily are composed of the canonical 35-residue motif, while the PLS subfamily is composed of trimers of motifs of various lengths and additional C-terminal domains (Lurin et al., 2004). The P-class PPR proteins have been shown to be involved in transcript stability and intron splicing, while most PLS proteins are implicated in RNA editing (Takenaka et al., 2013; Barkan and Small, 2014).
In Arabidopsis, whereas the majority of genes for mitochondrial proteins have been transferred to the nucleus and must be translated in the cytosol and redirected to the organelle, the mitochondrial genome has retained around 65 functional genes encoding proteins as well as tRNAs and ribosomal RNAs (Richardson et al., 2013). Apart from some ribosomal proteins and enzymes involved in the maturation of cytochrome c, the encoded proteins are subunits of complexes involved in oxidative phosphorylation located in the inner membrane. Of these, complex I (NADH-ubiquinone oxidoreductase) is considered the main entrance point for electrons into the mitochondrial respiratory chain. In plants, it is composed of more than 40 subunits, nine encoded by mitochondrial genes (nad1–nad4, nad4L, nad5–nad7, and nad9) and the others by nuclear genes. Most of the mitochondrially encoded subunits are part of the membrane arm, with the exception of Nad7 and Nad9, which belong to the hydrophilic peripheral arm (Peters et al., 2013). The assembly of subunits of nuclear and mitochondrial origins to form a functional complex requires complex mechanisms to coordinate the expression of genes from two physically separate genetic systems (Barkan, 2011) and a tight control of the processing of the mitochondrial transcripts by nuclearly encoded RNA-binding cofactors.
This is complicated by the fact that, in most angiosperms, several nad genes (nad1, nad2, and nad5) have been split and scattered around the mitochondrial chromosome. This gene arrangement is conserved between dicots and monocots, suggesting that it arose before these two groups separated (i.e. at least 140 million years ago; Malek and Knoop, 1998). The expression of the nad genes involves a combination of cis- and trans-splicing events that remove group II introns from the transcripts (Bonen, 2008). Several RNA-binding proteins are known to be involved in mitochondrial splicing events in nad transcripts: ORGANELLE TRANSCRIPT PROCESSING43 (OTP43; trans-splicing of nad1 intron 1; Falcon de Longevialle et al., 2007), ABSCISIC ACID OVERLY SENSITIVE5 (cis-splicing of nad2 intron 3; Liu et al., 2010), RCC1/UV-B RESISTANCE8/GUANINE NUCLEOTIDE EXCHANGE FACTOR3 (RUG3; (trans-splicing of nad2 intron 2 and cis-splicing of intron 3; Kühn et al., 2011), NUCLEAR MALE STERILE1 (cis-splicing of nad4 intron 1; Brangeon et al., 2000), BUTHIONINE SULFOMIXINE-INSENSITIVE ROOTS6 (BIR6; cis-splicing of nad7 intron 1; Koprivova et al., 2010), and other less-specific factors such as PUTATIVE MITOCHONDRIAL RNA HELICASE2 (PMH2; Köhler et al., 2010), maturases (nMAT1, nMAT2, and nMAT4; Keren et al., 2009, 2012; Brown et al., 2014; Cohen et al., 2014), and the MITOCHONDRIAL CHLOROPLAST RNA SPLICING ASSOCIATED FACTOR-like SPLICING FACTOR1 (mCSF1; Zmudjak et al., 2013) affecting numerous introns.
The nad5 mRNA is composed of five exons, derived from three transcription units (Knoop et al., 1991; Pereira de Souza et al., 1991; Bonen, 2008; Elina and Brown, 2010). Assembly of the mRNA requires two cis-splicing and two trans-splicing events. The order in which these steps are performed is critical to generate a functional nad5 mRNA, and failure to do so can generate mis-splicing of the second intron (Elina and Brown, 2010; Brown et al., 2014). Surprisingly, although several nad5 editing factors are known in Physcomitrella spp. and higher plants (Ohtani et al., 2010; Sosso et al., 2012; Toda et al., 2012; Verbitskiy et al., 2012), no factor specifically involved in nad5 splicing has been described so far.
In this study, we show that the PPR proteins OTP439 and TANG2 participate in the trans-splicing of nad5 introns 2 and 3, respectively. In both cases, the lack of splicing of nad5 leads to reduced levels of functional complex I in mitochondria. This work brings new information about the splicing of the complex nad5 transcript in plants and provides indirect or surrogate nad5 mutants useful for the study of the assembly of complex I.
RESULTS
Phenotypes of the tang2 and otp439 Mutants
The genes TANG2 (At1g19290) and OTP439 (At3g48810) are predicted to encode P-type PPR proteins comprising 20 and 16 conserved PPR motifs, respectively (Fig. 1, A and B). TANG2 was originally found to be one of thousands of genes whose expression was rapidly altered in response to Glc (Li et al., 2006). OTP439 was identified through a Genevestigator (https://www.genevestigator.ethz.ch) search for other sugar-inducible genes encoding PPR proteins. As several complex I mutants were reported to be sensitive to sugar (Falcon de Longevialle et al., 2007; Meyer et al., 2009; Zhu et al., 2012a, 2012b), we investigated the possible involvement of these two genes in complex I biogenesis. For this purpose, we obtained transfer DNA (T-DNA) insertion lines. The line SALK_003139 (tang2) contained the T-DNA insert within the coding region, 1,436 bp upstream of the stop codon (Fig. 1A). The line SALK_089911 (otp439) contained the T-DNA insert within the PPR repeat region, 663 bp downstream of the start codon (Fig. 1B).
Figure 1.
Gene model, T-DNA insertion, and visible phenotypes of otp439 and tang2 mutants. A and B, The T-DNA insertion in line SALK_003139 (named tang2) is located in the 11th PPR motif of the gene (A), and the insertion in line SALK_089911 (named otp439) is in the fifth PPR motif of the gene (B). C and D, The otp439 homozygous mutants develop slightly more slowly than the wild type (Col-0) but do not display any obvious phenotype (8-h light/16-h dark photoperiod). The tang2 homozygous mutants grow very slowly under 8-h light/16-h dark (C) or 16-h light/8-h dark (D) photoperiod and display dark curled foliage as compared with Col-0 and the complemented line tang2COM (D). The various PPR motifs are identified and labeled as done previously (Lurin et al., 2004).
When grown under an 8-h light/16-h dark photoperiod, otp439 only had a mild growth phenotype while tang2 exhibited very delayed growth as compared with the wild type (Fig. 1C). Under a 16-h light/8-h dark photoperiod, tang2 and otp439 mutants germinated 5 and 2 d later, respectively, than the wild type. Six-week-old tang2 plants displayed dark curled foliage (Fig. 1D), reminiscent of other complex I mutants such as otp43, NADH dehydrogenase (ubiquinone) fragment S subunit4, and rpoTmp (Falcon de Longevialle et al., 2007; Meyer et al., 2009; Kühn et al., 2011).
Complementation
Both tang2 and otp439 mutants were transformed with genomic DNA fragments carrying their respective wild-type genes, and several complemented lines were identified. For each mutant, one complemented line was used in further studies: both tang2COM and otp439COM germinated within hours of the wild type, showing that this trait had been rescued. Furthermore, tang2COM showed restored morphological and growth phenotypes (Fig. 1, C and D). Expression of TANG2 and OTP439 was confirmed by quantitative PCR in the complemented plants (data not shown).
TANG2 and OTP439 Are Localized in Mitochondria
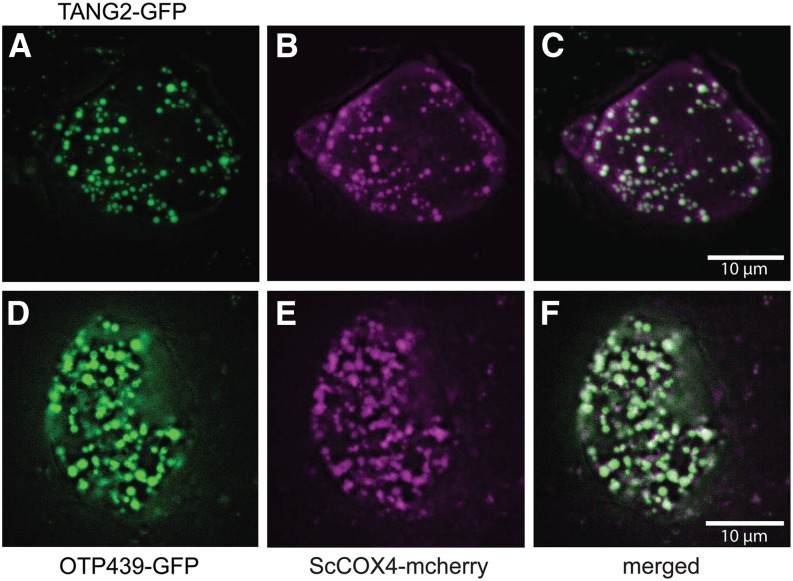
To check the targeting of TANG2 and OTP439, GFP fusions were biolistically transformed into Arabidopsis cells together with a mitochondria-specific protein (cytochrome c oxidase subunit4 [COX4] from Saccharomyces cerevisiae) fused to the red fluorescent protein. The overlay of the green and red fluorescence shows that both TANG2 and OTP439 are localized to the mitochondrion (Fig. 2).
Figure 2.
Subcellular localization of the PPR proteins TANG2 and OTP439 by GFP tagging. GFP fusions were biolistically transformed into Arabidopsis cells (A and D) together with a mitochondria-specific protein (COX4 from S. cerevisiae) fused to the red fluorescent protein (B and E). C and F show overlays of the green and red fluorescence images.
TANG2 and OTP439 Are Involved in nad5 Splicing
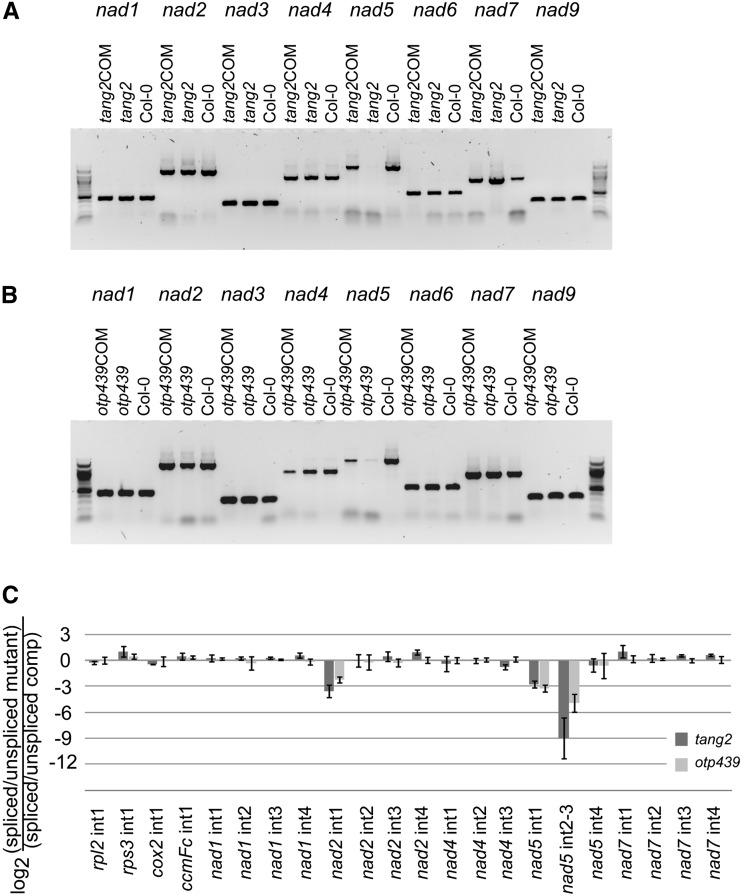
As both genes encode P-class PPR proteins and many of these proteins are involved in RNA processing (Barkan and Small, 2014), we performed reverse transcription (RT)-PCR to analyze mitochondrial transcripts encoding subunits of complex I in tang2, otp439, their complemented lines, and Columbia-0 (Col-0). Figure 3 shows that the mature, fully processed nad5 transcript was weakly detected in tang2 (Fig. 3A) and otp439 (Fig. 3B) as compared with Col-0 and complemented lines, whereas other nad transcripts accumulated normally, suggesting that the transcription, processing, or stability of nad5 RNA is defective in these mutants. As several other PPR proteins are known to be involved in transcript-specific splicing (Falcon de Longevialle et al., 2010; Colas des Francs-Small and Small, 2014), and in particular splicing of transcripts encoding complex I subunits, the mutants were screened for splicing defects by a quantitative PCR assay (Falcon de Longevialle et al., 2007; Fig. 3C). For this test, two sets of primers are used, one set specifically amplifying spliced RNA and the other set specifically amplifying unspliced RNA. Splicing efficiency is calculated as a ratio of spliced to unspliced forms of each transcript in the mutant normalized to the same ratio in the complemented plants (Falcon de Longevialle et al., 2007; Koprivova et al., 2010; Colas des Francs-Small et al., 2012). Due to its small size, nad5 exon 3 was not amplified individually; for this initial screen, the primers amplifying the spliced transcript were placed on exons 2 and 4, and thus the splicing efficiencies of introns 2 and 3 are presented together in the histogram as intron 2-3 (Fig. 3C). Both mutants exhibited defects in the splicing of nad5 introns (introns 1 and 2-3), but the splicing efficiency of intron 2-3 was about 8-fold lower for tang2 than for otp439. Both also appeared affected in the splicing of nad2 intron 1, but the latter could be a pleiotropic effect, as it has been reported previously for mutants impaired in complex I (Falcon de Longevialle et al., 2007; Koprivova et al., 2010). The splicing efficiency of other transcripts was not significantly altered in either mutant (Fig. 3C).
Figure 3.
A and B, Transcript analysis. RT-PCR is shown for nad1, nad2, nad3, nad4, nad5, nad6, nad7, and nad9 transcripts in tang2, tang2COM, and Col-0 plants (A) and in otp439, otp439COM, and Col-0 plants (B). C, Quantitative RT-PCR of intron-containing mitochondrial transcripts. The histogram shows the log2 ratio of spliced to unspliced forms for each transcript in the mutants as compared with the corresponding complemented plants. Each value is a mean of three biological replicates.
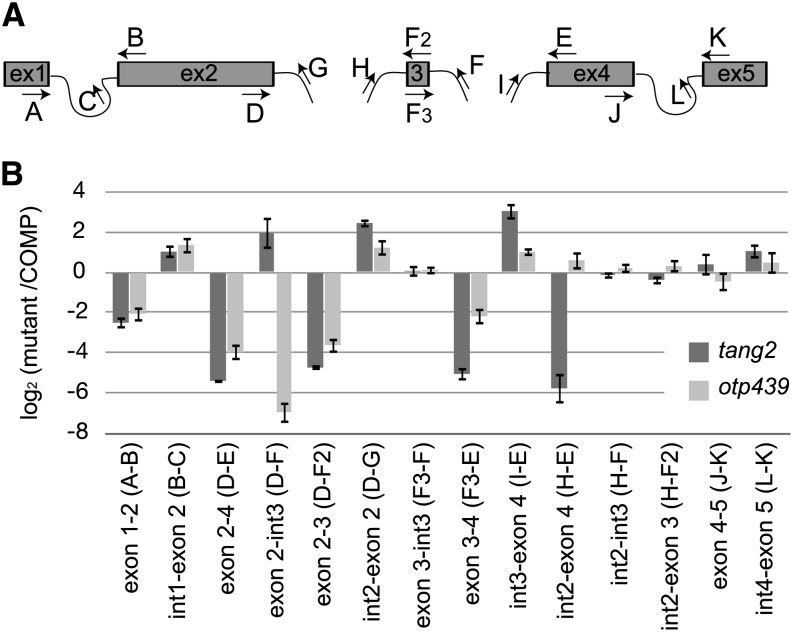
The complex process of nad5 maturation (Fig. 4A) led us to check splicing intermediates in the mutants. Assembly of the mRNA, comprising five exons, requires two cis-splicing events to join exons 1 and 2 on the one hand and exons 4 and 5 on the other hand (Elina and Brown, 2010; Brown et al., 2014). Two trans-splicing events are required to join the 22-nucleotide-long exon 3 to the other two parts and obtain a functional nad5 transcript (Knoop et al., 1991; Pereira de Souza et al., 1991). The order in which these trans-splicing steps happen is essential; intron 3 splicing (joining exons 3 and 4) must be performed before intron 2 splicing (joining exons 2 and 3) can be properly executed. Extensive mis-splicing of intron 2 has been reported in monocots and dicots (Elina and Brown, 2010; Brown et al., 2014). In an attempt to determine the specific roles of TANG2 and OTP439 in nad5 splicing, various fragments within the region encompassing exons 2, 3, and 4 (Fig. 4A, primers D and E) were amplified. Analysis of splicing intermediates (Fig. 4B) shows that the accumulation levels of spliced exon 2-3 are comparable to those of exon 2-4 in both mutants (with lower levels for tang2 than otp439). This result, coupled with the high accumulation of unspliced forms of introns 2 and 3 (higher in tang2 than in otp439), confirms that these are true splicing defects. In tang2, the quasi-absence of spliced exon 3-4 and the strong accumulation of unspliced intron 3 suggest that TANG2 is involved in splicing this intron. The inefficient splicing of intron 3 is likely to inhibit the splicing of intron 2, which cannot be processed correctly, as intron 3 is still present in the pre-mRNA (Elina and Brown, 2010). This is confirmed by the increase of the splicing intermediate exon2-intron3 (Fig. 4B, primers D–F) in tang2. The situation is different in otp439; there, the main defect lies in intron 2 splicing, suggesting that it is the primary target of OTP439.
Figure 4.
Detailed analysis of nad5 splicing. A, Diagram of the splicing events necessary for generating a translatable nad5 transcript and positions of the primers used. B, Quantitative RT-PCR of the mature and unspliced mRNAs of individual nad5 exons in tang2 and otp439 analyzed using various combinations of the primers described in A and in Supplemental Table S1. The histogram shows the log2 values of the ratios of mutants to complemented plants for each transcript. Each value is a mean of three biological replicates.
Splicing Defects Result in Complex I Deficiency in tang2 and otp439
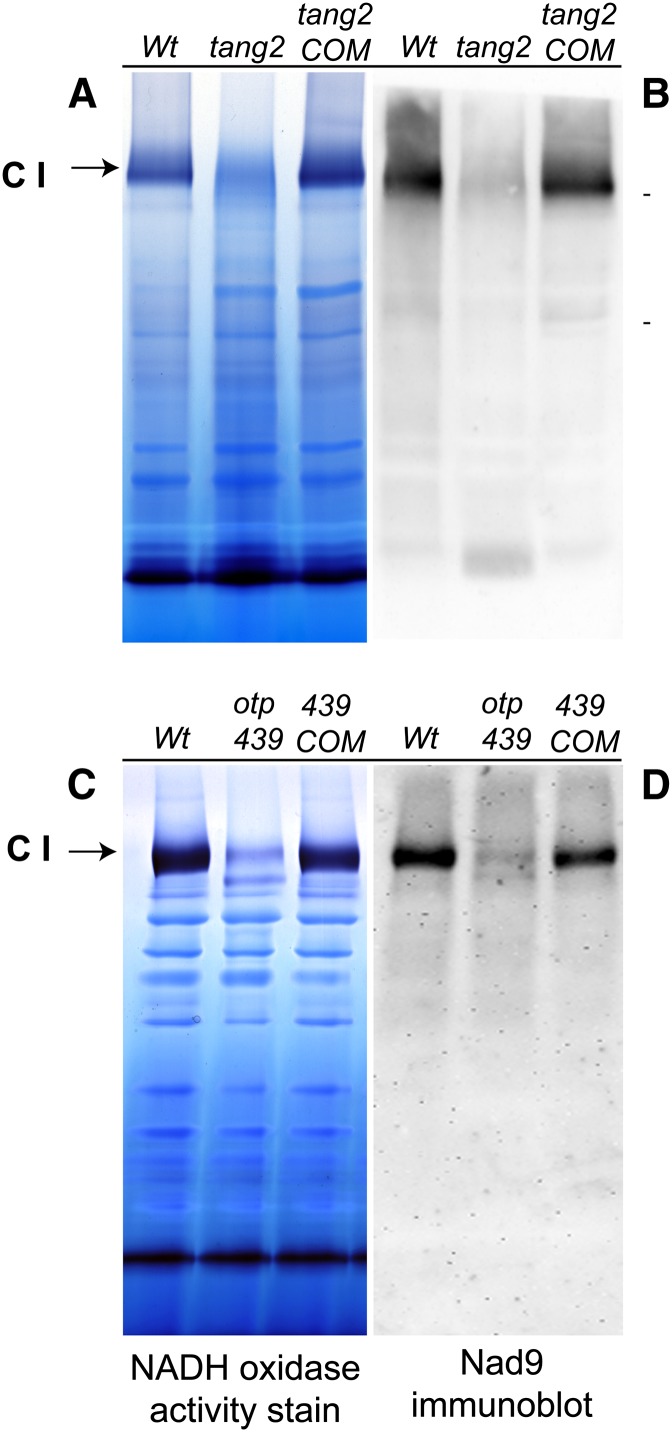
To determine whether the nad5 splicing defects affect complex I accumulation and activity in the mutants, mitochondrial membrane proteins were analyzed by blue native (BN)-PAGE and subsequent NADH oxidase activity staining. As shown in Figure 5, NADH oxidase activity at the position expected for complex I was almost undetectable in tang2 (Fig. 5A) compared with Col-0 and tang2COM. A similar BN-PAGE gel was blotted and probed with a Nad9 antibody (Fig. 5B), proving that tang2 mitochondria have very little assembled complex I. Similar gels and blots (Fig. 5, C and D) show that the reduction was not as severe for otp439 but was still very noticeable as compared with Col-0 and otp439COM.
Figure 5.
BN-PAGE and immunoblot analysis of mitochondrial proteins from tang2 (A and B) and otp439 (C and D) mutants. A and C represent BN gels stained for the NADH oxidase activity, and B and D show the polyvinylidene difluoride membranes probed with an anti-Nad9 antibody. Complex I (CI) is indicated by the arrows. Wt, Wild type.
DISCUSSION
Introns are rather common in land plant mitochondrial genomes, with a total of 23 in Arabidopsis, 25 in Physcomitrella spp., and 32 in Marchantia polymorpha (for review, see Bonen, 2008). Although the mitochondrial genomes of Physcomitrella spp. and M. polymorpha also contain several group I introns, the vast majority of the introns in flowering plants (with the exception of the cox1 intron in some species) belong to group II, with 23 in Arabidopsis and rice (Oryza sativa; Bonen, 2008). These introns seem to have lost their self-splicing ability, thus necessitating the help of maturases such as the nuclearly encoded nMAT1, nMAT2, nMAT3, and nMAT4 and possibly the mitochondrial MatR, whose function is still elusive (Keren et al., 2009, 2012; Brown et al., 2014; Cohen et al., 2014). Plant mitochondrial DNA is renowned for its high recombination rates and its ability to integrate foreign DNA sequences, explaining the difficulty of retracing the evolutionary story of group II introns (Bonen, 2008). All trans-splicing introns in plant mitochondria seem to have arisen via genomic recombination of monopartite introns in vascular plants, as ancestral cis-arranged introns are found for all of them in ferns, fern allies, or hornworts (Malek and Knoop, 1998). Gene fragmentation created the need for trans-splicing, a phenomenon that occurs for three genes (nad1, nad2, and nad5) encoding core subunits (i.e. homologous to the bacterial complex I subunits) of the respiratory complex I, whose sequences are very conserved (Meyer, 2012), stressing the need for a very reliable transcript maturation and splicing system. The complexity of nad5 transcript assembly (Fig. 4A) suggests the participation of numerous RNA-processing factors.
Our results show that the PPR proteins OTP439 and TANG2 are specifically involved in the trans-splicing of introns 2 and 3, respectively. For each trans-splicing event, the participation of two specific factors (at least) is likely to be required, each specifically binding and bringing together distant parts of the intron to be folded and subsequently spliced out. As nad5 introns 2 and 3 are both spliced in trans and introns 1 and 4 are spliced in cis, we can expect to find at least six specific factors for nad5 alone, as well as maturases and more general splicing factors. mCSF1, a CRM protein, is involved in splicing introns 1, 2, 3, and possibly 4 (Zmudjak et al., 2013). PPR proteins, maturases, CRM proteins, as well as the PMH2 helicase, an RNA chaperone required for the formation or maintenance of complex RNA secondary structures of introns, are likely to be part of a heteromultimeric splicing complex, as suggested for mitochondrial (Köhler et al., 2010) and chloroplast group IIB (Asakura et al., 2008) introns.
The splicing defects of nad5 introns 2 (in otp439) and 3 (in tang2) are sufficient to explain the dramatic reduction of fully processed nad5 transcripts, leading to a reduced accumulation of complex I. This phenomenon has been reported for other mutants, such as otp43 (Falcon de Longevialle et al., 2007), bir6 (Koprivova et al., 2010), mtsf1 (Haïli et al., 2013), nMat1 (Keren et al., 2012), nMat2 (Keren et al., 2009), nMat4 (Cohen et al., 2014), mcsf1 (Zmudjak et al., 2013), and indh (Wydro et al., 2013), as well as the Nicotiana sylvestris mutant cms2 (Gutierres et al., 1997) and the maize (Zea mays) nonchromosomal stripe1 (Karpova and Newton, 1999). The molecular defect in tang2 is much stronger than that of otp439, and this is reflected by the quantity of the assembled complex I in the mutants, which is very likely the reason behind the discrepancy between their phenotypes.
As mutants in mitochondrial genes are difficult to obtain and currently impossible to deliberately create, these two indirect complex I mutants are an important addition to the recent list of such surrogate mutants (Colas des Francs-Small and Small, 2014) and it will be useful to further explore the assembly of complex I in Arabidopsis (Meyer et al., 2011).
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) mutants (tang2 [SALK_003139] and otp439 [SALK_089911]) were obtained from the Nottingham Arabidopsis Stock Centre. The seeds were vernalized at 4°C for 2 d in the dark and grown at 22°C either under a 16-h light/8-h dark or an 8-h light/16-h dark photoperiod.
Complementation Tests
A genomic DNA fragment containing the entire TANG2 (At1g19290) coding region with 1.5-kb upstream and 1-kb downstream sequences was inserted into the binary vector pCAMBIA1300. Similarly, a genomic DNA fragment containing the entire OTP439 (At3g48810) coding region with 1.5-kb upstream and 1-kb downstream sequences was inserted into the binary vector pCAMBIA1390. These plasmids were introduced into the tang2 and otp439 mutants, respectively, using Agrobacterium tumefaciens GV3101, and transformants were selected on hygromycin-containing medium.
Subcellular Localization of TANG2 and OTP439 by GFP Tagging
The first 300 bp of the coding sequences of TANG2 and OTP439 were amplified using the Expand High Fidelity PCR system (Roche Diagnostics). The PCR products were cloned into Gateway vector pDONR207 (Invitrogen) and sequenced. The entry vector and a Gateway cloning cassette (Carrie et al., 2009) were recombined to clone the targeting sequences of TANG2 and OTP439 in frame with the coding region of the GFP. For colocalization studies, the mitochondrial targeting sequence of Saccharomyces cerevisiae ScCox4 fused to mCherry in pBIN20 (Nelson et al., 2007) was used as a mitochondrial control. The fusion constructs were biolistically transformed into cultured Arabidopsis cells (ecotype Landsberg erecta). After bombardment, the Arabidopsis cell suspension was placed in the dark at 22°C. Fluorescence images were obtained 24 h after transformation using an Olympus BX61 epifluorescence microscope with excitation wavelengths of 460/480 nm (GFP) and 535/555 nm (mCherry) and emission wavelengths of 495 to 540 nm (GFP) and 570 to 625 nm (mCherry). Subsequent images were captured using cell imaging software.
Mitochondrial RNA Transcript Analysis
Total leaf RNA was isolated from 6-week-old rosette leaves using the Plant RNeasy extraction kit (Qiagen), and genomic DNA was removed using TURBO DNase (Ambion). RT was performed on 3 μg of RNA using SuperScript III (Invitrogen) and random hexamer primers as described previously (Falcon de Longevialle et al., 2007). RT-PCR was performed as described previously (Falcon de Longevialle et al., 2007). The sequences of the primers used are given in Supplemental Table S1.
To quantify the splicing of mitochondrial mRNAs, primers were designed to amplify 100- to 200-bp regions spanning intron-exon junctions (unspliced forms) or splice junctions (spliced forms) of each gene. The primers used for the splicing test quantitative RT-PCR assays were described previously (Koprivova et al., 2010; Haïli et al., 2013). Quantitative RT-PCR was performed using LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics) and a Roche LightCycler 480 real-time PCR system with the following thermal cycling program: 95°C for 10 min, followed by 45 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s. The data were analyzed using the LightCycler 480 software version 1.5 (Roche Diagnostics). Three independent experiments were performed, and each sample was run in triplicate. Nuclear 18S ribosomal RNA was used as an internal control.
Mitochondrial Isolation, BN-PAGE, and Immunoblot Analysis
Mitochondria were isolated from 8-week-old rosette leaves, and BN-PAGE was performed as described previously (Falcon de Longevialle et al., 2007). Proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad), in cathode buffer for 15 h at 40 mA, using a Bio-Rad Mini Transblot Cell. Western-blot analysis was performed using an anti-Nad9 antibody (Lamattina et al., 1993) and a goat anti-rabbit secondary antibody conjugated to horseradish peroxidase, and the results were subsequently revealed using enhanced chemiluminescence reagents (GE Healthcare).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. List of primers used in this work.
Supplementary Material
Glossary
- T-DNA
transfer DNA
- RT
reverse transcription
- Col-0
Columbia-0
- BN
blue native
- PPR
pentatricopeptide repeat
Footnotes
This work was supported by the Australian Research Council Centre of Excellence (grant no. CE0561495), the Department of Education, Science and Training International Science Linkages (grant no. CG120098), the Biotechnology and Biological Sciences Research Council (grant no. BB/C515620), the John Innes Growth and Development Underpinning Yield Strategic Programme (grant no. BB/J004588/1), and the National Natural Science Foundation of China (grant no. 91017014).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Andersson SGE, Karlberg O, Canbäck B, Kurland CG. (2003) On the origin of mitochondria: a genomics perspective. Philos Trans R Soc Lond B Biol Sci 358: 165–177, discussion 177–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura Y, Barkan A. (2006) Arabidopsis orthologs of maize chloroplast splicing factors promote splicing of orthologous and species-specific group II introns. Plant Physiol 142: 1656–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura Y, Bayraktar OA, Barkan A. (2008) Two CRM protein subfamilies cooperate in the splicing of group IIB introns in chloroplasts. RNA 14: 2319–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. (2011) Expression of plastid genes: organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol 155: 1520–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I. (2014) Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol 65: 415–442 [DOI] [PubMed] [Google Scholar]
- Bentolila S, Heller WP, Sun T, Babina AM, Friso G, van Wijk KJ, Hanson MR. (2012) RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc Natl Acad Sci USA 109: E1453–E1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L. (2008) Cis- and trans-splicing of group II introns in plant mitochondria. Mitochondrion 8: 26–34 [DOI] [PubMed] [Google Scholar]
- Brangeon J, Sabar M, Gutierres S, Combettes B, Bove J, Gendy C, Chétrit P, Des Francs-Small CC, Pla M, Vedel F, et al. (2000) Defective splicing of the first nad4 intron is associated with lack of several complex I subunits in the Nicotiana sylvestris NMS1 nuclear mutant. Plant J 21: 269–280 [DOI] [PubMed] [Google Scholar]
- Brown G, Colas des Francs-Small C, Ostersetzer O. (2014) Group-II intron splicing factors in higher-plants mitochondria. Front Plant Physiol Sci 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie C, Kühn K, Murcha MW, Duncan O, Small ID, O’Toole N, Whelan J. (2009) Approaches to defining dual-targeted proteins in Arabidopsis. Plant J 57: 1128–1139 [DOI] [PubMed] [Google Scholar]
- Cohen S, Zmudjak M, Colas des Francs-Small C, Malik S, Shaya F, Keren I, Belausov E, Many Y, Brown GG, Small I, et al. (2014) nMAT4, a maturase factor required for nad1 pre-mRNA processing and maturation, is essential for holocomplex I biogenesis in Arabidopsis mitochondria. Plant J 78: 253–268 [DOI] [PubMed] [Google Scholar]
- Colas des Francs-Small C, Kroeger T, Zmudjak M, Ostersetzer-Biran O, Rahimi N, Small I, Barkan A. (2012) A PORR domain protein required for rpl2 and ccmF(C) intron splicing and for the biogenesis of c-type cytochromes in Arabidopsis mitochondria. Plant J 69: 996–1005 [DOI] [PubMed] [Google Scholar]
- Colas des Francs-Small C, Small I. (2014) Surrogate mutants for studying mitochondrially encoded functions. Biochimie 100: 234–242 [DOI] [PubMed] [Google Scholar]
- Elina H, Brown GG. (2010) Extensive mis-splicing of a bi-partite plant mitochondrial group II intron. Nucleic Acids Res 38: 996–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon de Longevialle A, Meyer EH, Andrés C, Taylor NL, Lurin C, Millar AH, Small ID. (2007) The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 intron 1 in Arabidopsis thaliana. Plant Cell 19: 3256–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon de Longevialle A, Small ID, Lurin C. (2010) Nuclearly encoded splicing factors implicated in RNA splicing in higher plant organelles. Mol Plant 3: 691–705 [DOI] [PubMed] [Google Scholar]
- Gutierres S, Sabar M, Lelandais C, Chétrit P, Diolez P, Degand H, Boutry M, Vedel F, de Kouchkovsky Y, De Paepe R. (1997) Lack of mitochondrial and nuclear-encoded subunits of complex I and alteration of the respiratory chain in Nicotiana sylvestris mitochondrial deletion mutants. Proc Natl Acad Sci USA 94: 3436–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haïli N, Arnal N, Quadrado M, Amiar S, Tcherkez G, Dahan J, Briozzo P, Colas des Francs-Small C, Vrielynck N, Mireau H. (2013) The pentatricopeptide repeat MTSF1 protein stabilizes the nad4 mRNA in Arabidopsis mitochondria. Nucleic Acids Res 41: 6650–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova OV, Newton KJ. (1999) A partially assembled complex I in NAD4-deficient mitochondria of maize. Plant J 17: 511–521 [Google Scholar]
- Keren I, Bezawork-Geleta A, Kolton M, Maayan I, Belausov E, Levy M, Mett A, Gidoni D, Shaya F, Ostersetzer-Biran O. (2009) AtnMat2, a nuclear-encoded maturase required for splicing of group-II introns in Arabidopsis mitochondria. RNA 15: 2299–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Tal L, Colas des Francs-Small C, Araújo WL, Shevtsov S, Shaya F, Fernie AR, Small I, Ostersetzer-Biran O. (2012) nMAT1, a nuclear-encoded maturase involved in the trans-splicing of nad1 intron 1, is essential for mitochondrial complex I assembly and function. Plant J 71: 413–426 [DOI] [PubMed] [Google Scholar]
- Knoop V, Schuster W, Wissinger B, Brennicke A. (1991) Trans splicing integrates an exon of 22 nucleotides into the nad5 mRNA in higher plant mitochondria. EMBO J 10: 3483–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler D, Schmidt-Gattung S, Binder S. (2010) The DEAD-box protein PMH2 is required for efficient group II intron splicing in mitochondria of Arabidopsis thaliana. Plant Mol Biol 72: 459–467 [DOI] [PubMed] [Google Scholar]
- Koprivova A, Colas des Francs-Small C, Calder G, Mugford ST, Tanz S, Lee BR, Zechmann B, Small I, Kopriva S. (2010) Identification of a pentatricopeptide repeat protein implicated in splicing of intron 1 of mitochondrial nad7 transcripts. J Biol Chem 285: 32192–32199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger TS, Watkins KP, Friso G, van Wijk KJ, Barkan A. (2009) A plant-specific RNA-binding domain revealed through analysis of chloroplast group II intron splicing. Proc Natl Acad Sci USA 106: 4537–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn K, Carrie C, Giraud E, Wang Y, Meyer EH, Narsai R, Colas des Francs-Small C, Zhang B, Murcha MW, Whelan J. (2011) The RCC1 family protein RUG3 is required for splicing of nad2 and complex I biogenesis in mitochondria of Arabidopsis thaliana. Plant J 67: 1067–1080 [DOI] [PubMed] [Google Scholar]
- Lamattina L, Gonzalez D, Gualberto J, Grienenberger JM. (1993) Higher plant mitochondria encode an homologue of the nuclear-encoded 30-kDa subunit of bovine mitochondrial complex I. Eur J Biochem 217: 831–838 [DOI] [PubMed] [Google Scholar]
- Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW. (2006) Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res 16: 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, He J, Chen Z, Ren X, Hong X, Gong Z. (2010) ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. Plant J 63: 749–765 [DOI] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al. (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek O, Knoop V. (1998) Trans-splicing group II introns in plant mitochondria: the complete set of cis-arranged homologs in ferns, fern allies, and a hornwort. RNA 4: 1599–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, Herrmann RG. (1998) Gene transfer from organelles to the nucleus: how much, what happens, and why? Plant Physiol 118: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH. (2012) Proteomic investigations of complex I composition: how to define a subunit? Front Plant Sci 3: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH, Solheim C, Tanz SK, Bonnard G, Millar AH. (2011) Insights into the composition and assembly of the membrane arm of plant complex I through analysis of subcomplexes in Arabidopsis mutant lines. J Biol Chem 286: 26081–26092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH, Tomaz T, Carroll AJ, Estavillo G, Delannoy E, Tanz SK, Small ID, Pogson BJ, Millar AH. (2009) Remodeled respiration in ndufs4 with low phosphorylation efficiency suppresses Arabidopsis germination and growth and alters control of metabolism at night. Plant Physiol 151: 603–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A. (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Ohtani S, Ichinose M, Tasaki E, Aoki Y, Komura Y, Sugita M. (2010) Targeted gene disruption identifies three PPR-DYW proteins involved in RNA editing for five editing sites of the moss mitochondrial transcripts. Plant Cell Physiol 51: 1942–1949 [DOI] [PubMed] [Google Scholar]
- Pereira de Souza A, Jubier MF, Delcher E, Lancelin D, Lejeune B. (1991) A trans-splicing model for the expression of the tripartite nad5 gene in wheat and maize mitochondria. Plant Cell 3: 1363–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K, Belt K, Braun HP. (2013) 3D gel map of Arabidopsis complex I. Front Plant Sci 4: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Allen JF. (2003) Genomics and chloroplast evolution: what did cyanobacteria do for plants? Genome Biol 4: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AO, Rice DW, Young GJ, Alverson AJ, Palmer JD. (2013) The “fossilized” mitochondrial genome of Liriodendron tulipifera: ancestral gene content and order, ancestral editing sites, and extraordinarily low mutation rate. BMC Biol 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small ID, Peeters N. (2000) The PPR motif: a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 25: 46–47 [DOI] [PubMed] [Google Scholar]
- Sosso D, Mbelo S, Vernoud V, Gendrot G, Dedieu A, Chambrier P, Dauzat M, Heurtevin L, Guyon V, Takenaka M, et al. (2012) PPR2263, a DYW-subgroup pentatricopeptide repeat protein, is required for mitochondrial nad5 and cob transcript editing, mitochondrion biogenesis, and maize growth. Plant Cell 24: 676–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka M, Zehrmann A, Verbitskiy D, Härtel B, Brennicke A. (2013) RNA editing in plants and its evolution. Annu Rev Genet 47: 335–352 [DOI] [PubMed] [Google Scholar]
- Takenaka M, Zehrmann A, Verbitskiy D, Kugelmann M, Härtel B, Brennicke A. (2012) Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc Natl Acad Sci USA 109: 5104–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Fujii S, Noguchi K, Kazama T, Toriyama K. (2012) Rice MPR25 encodes a pentatricopeptide repeat protein and is essential for RNA editing of nad5 transcripts in mitochondria. Plant J 72: 450–460 [DOI] [PubMed] [Google Scholar]
- Verbitskiy D, Zehrmann A, Härtel B, Brennicke A, Takenaka M. (2012) Two related RNA-editing proteins target the same sites in mitochondria of Arabidopsis thaliana. J Biol Chem 287: 38064–38072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydro MM, Sharma P, Foster JM, Bych K, Meyer EH, Balk J. (2013) The evolutionarily conserved iron-sulfur protein INDH is required for complex I assembly and mitochondrial translation in Arabidopsis [corrected]. Plant Cell 25: 4014–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Dugardeyn J, Zhang C, Takenaka M, Kühn K, Craddock C, Smalle J, Karampelias M, Denecke J, Peters J, et al. (2012) SLO2, a mitochondrial pentatricopeptide repeat protein affecting several RNA editing sites, is required for energy metabolism. Plant J 71: 836–849 [DOI] [PubMed] [Google Scholar]
- Zhu Q, Meyer EH, Van Der Straeten D. (2012) Functional analysis of SLO2 provides new insight into the role of plant PPR proteins. Plant Signal Behav 7: 1209–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudjak M, Colas des Francs-Small C, Keren I, Shaya F, Belausov E, Small I, Ostersetzer-Biran O. (2013) mCSF1, a nucleus-encoded CRM protein required for the processing of many mitochondrial introns, is involved in the biogenesis of respiratory complexes I and IV in Arabidopsis. New Phytol 199: 379–394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.