Abstract
Vacuolar sorting receptors bind cargo ligands early in the secretory pathway and show that multivesicular body-vacuole fusion requires a Rab5/Rab7 GTPase conversion with consequences for retromer binding.
To serve the purposes of controlled protein turnover, eukaryotic cells compartmentalize the required acid hydrolases in specialized digestive organelles: lysosomes in animals and vacuoles in yeasts and plants. Therefore, a reliable system must be in operation to prevent such proteolytic enzymes being released at the cell surface. Such a mechanism requires that acid hydrolases be identified and diverted away from the secretory flow to the plasma membrane (PM). This process is facilitated by receptors that recognize specific motifs in the hydrolases that are absent in secretory proteins. The most well-known example of this is the mannosyl 6-phosphate receptor (MPR), which is responsible for the sorting of lysosomal enzymes; indeed, it has become a paradigm for protein sorting in most cell biology textbooks. It entails the recognition of phosphomannan cargo ligands by MPRs in the trans-Golgi network (TGN) followed by the sequestration of the MPR-ligand complexes into specific transport vectors (clathrin-coated vesicles [CCVs]). These are then transported to an endosomal compartment (the early endosome [EE]) having a more acidic pH than the TGN, thereby causing the ligands to separate from the MPRs. The MPRs are subsequently recycled back to the TGN via retromer-coated carriers for another round of trafficking (for review, see Braulke and Bonifacino, 2009; Seaman, 2012).
Many plant scientists support a scenario for the sorting of soluble vacuolar proteins and the trafficking of their receptors (vacuolar sorting receptors [VSRs]) that closely resembles that of the MPR system of mammalian cells (Hwang, 2008; De Marcos Lousa et al., 2012; Kang et al., 2012; Sauer et al., 2013; Xiang et al., 2013). This working model is based on three key observations: (1) VSRs were first identified in detergent-solubilized CCV fractions isolated from developing pea (Pisum sativum) cotyledons; (2) CCVs are regularly seen budding off the TGN in thin-sectioned plant cells; and (3) depending on the organism, VSRs and VSR-reporter constructs are found concentrated either in the TGN or in multivesicular prevacuolar compartments (PVCs) under steady-state conditions (Robinson and Pimpl, 2014a, 2014b, and refs. therein). Unfortunately, information on VSRs has not been obtained from a single experimental system. Although much work on Arabidopsis (Arabidopsis thaliana) VSR mutants has been published (for review, see De Marcos Lousa et al., 2012) and the majority of immunogold electron microscopic localization experiments have been performed in Arabidopsis, the majority of the fluorescence localizations, particularly with regard to VSR trafficking, have been carried out by transient expression in tobacco (Nicotiana tabacum; agroinfiltration for leaves and electroporation for protoplasts). Nevertheless, it should be stressed that sorting motifs for acid hydrolases and their corresponding receptors in the three major eukaryotic organismal groups differ considerably (Robinson et al., 2012). In addition, the secretory and endocytic pathways of plant cells contrast significantly with mammalian cells, the most important distinctions being (1) the lack of an intermediate compartment between the endoplasmic reticulum (ER) and the Golgi apparatus in plants, (2) that plants have motile Golgi stacks rather than a perinuclear Golgi complex, and (3) the absence of an independent EE in plants, the function of which is assumed by the TGN (Contento and Bassham, 2012). While these differences do not automatically negate the validity of the above working model for VSR trafficking, they at least legitimize a more thorough analysis of the supporting data than has previously been the case (Robinson and Pimpl, 2014a, 2014b).
The principal issues at stake are as follows. Where do VSRs bind and release their cargo ligands? What is the actual mechanism resulting in the separation of secretory from vacuolar cargo molecules? What is/are the precise role(s) of TGN-derived CCVs? And where does retromer pick up VSRs and where are they delivered to? The impact of several new publications on these points of dispute is the subject of this article.
VSRs BIND CARGO LIGANDS IN THE ER: EVIDENCE AND CONSEQUENCES
Since immunogold electron microscopy has confirmed the presence of VSRs in CCVs at the TGN (Hinz et al., 2007, and refs. therein), it was thought that this is where vacuolar ligands became bound to their VSRs. While being morphologically in agreement with the situation in mammalian cells, this interpretation does not take into account the different biochemical premises for receptor-ligand interactions in the mammalian and plant systems. In mammalian cells, acid hydrolases are identified through a tertiary conformational motif (signal patch) in the cis-Golgi and then receive a secondary recognition signal: phosphorylation of terminal Man residues in N-oligosaccharide side chains (Braulke and Bonifacino, 2009). This signal, however, remains masked until the acid hydrolase arrives in the TGN, where it becomes unveiled, leading to ligand binding (i.e. the MPR-ligand interaction occurs immediately prior to sequestration into nascent CCVs; for details and pertinent literature, see Robinson et al., 2012). This process may seem complicated, but it ensures that receptor-ligand interactions cannot occur earlier in the secretory pathway than the TGN. In contrast, vacuolar cargo ligands in plants have primary sequence-sorting determinants (Neuhaus and Paris, 2005), so there is no a priori reason for their recognition to be delayed until entering the TGN.
Convincing evidence that VSR-ligand interactions are in fact initiated earlier than the TGN in the secretory pathway was recently published by Gershlick et al. (2014). These authors prepared reporter constructs having both an N-terminal vacuolar sorting signal (NPIR) and a C-terminal ER retention signal (HDEL). Although the authors did not take into account the possibility that dual-signal molecules might bind simultaneously to VSRs and the ER retrieval receptor endoplasmic reticulum retention defective2 (ERD2), HDEL/KDEL receptor, they nevertheless compared the transport of such dual signal reporters with reporters containing only either an HDEL retrieval signal or an NPIR vacuolar sorting signal as the sole sorting information. On the basis of their data, Gershlick et al. (2014) concluded that VSRs and the ER retrieval receptor ERD2 were in competition with one another for transport ligands, and since ERD2 binds to HDEL cargo in the cis-Golgi (Phillipson et al., 2001), it was inferred that this must be the location for VSR-ligand interactions as well.
That VSRs should meet their ligands early in the secretory pathway is actually not a novel finding, since there exist other reports that claim that VSR-ligand interaction in fact already starts in the ER (Watanabe et al., 2004; daSilva et al., 2005). The basic approach in these earlier studies was to trap VSR ligands in the early secretory pathway by expressing the luminal ligand-binding domain (LBD) of the VSR as a soluble protein that additionally carries an HDEL peptide for ER retrieval at its C terminus. The VSR(LBD)-HDEL construct would then cycle between the ER and the cis-Golgi. Although it was not possible to pinpoint the location of the initial VSR(LBD)-ligand interaction, in both cases vacuolar proteins accumulated in the ER lumen. However, more recently, it was shown that VSR-LBDs, which were directly anchored in the ER as fusion proteins between the LBD and the transmembrane domain of calnexin, an ER-resident protein (Niemes et al., 2010a), also caused VSR ligands to accumulate in the ER and prevented their delivery to the vacuole. This strongly suggests that native VSRs will also interact with their cargo ligands in the ER and do not need to be transported separately via bulk flow to the cis-Golgi for this to occur (for a comparison of the two possibilities, see Fig. 1). Indeed, since VSR-ligand interactions are positively influenced by Ca2+ (Watanabe et al., 2002) and the ER has much higher Ca2+ levels than the Golgi (Ordenes et al., 2012), it is hard to understand what would prevent their interaction in the ER lumen or, for that matter, in the confined space of a coat protein vesicle II (COPII) transport vesicle.
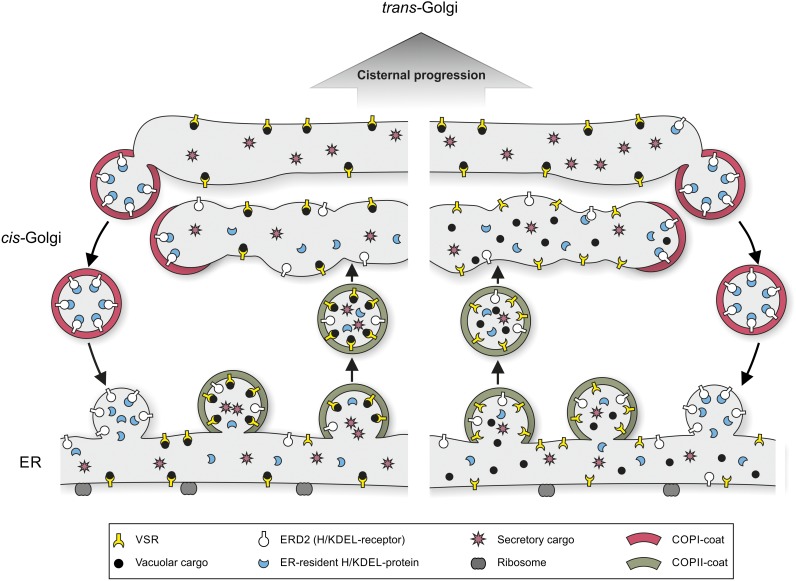
Figure 1.
Two possible scenarios for VSR-ligand interactions in the early secretory pathway. At the left, soluble vacuolar cargo molecules are already recognized by VSRs while still in the lumen of the ER. Receptor-ligand binding is favored by the high Ca2+ concentrations in the ER. In the version at the right, ligand binding to VSRs is delayed until the cis-Golgi cisternae, where the K(H)DEL-receptor scavenges ER-resident proteins that have inadvertently entered COPII transport vesicles. Whereas the latter are selectively sequestered into COPI vesicles for recycling back to the ER, VSR-ligand complexes are excluded and move up through the Golgi stack via cisternal maturation.
Several conclusions may be drawn on the knowledge that VSR-ligand binding starts in the ER (or cis-Golgi). (1) VSR-ligand binding is spatially separated from the sorting event that segregates vacuolar from secretory traffic. (2) High concentrations of VSRs do not faithfully reflect locations where ligand binding takes place. (3) If VSR-ligand complexes are exported out of the TGN in CCVs, as many plant scientists believe, then these complexes would naturally become concentrated at the TGN prior to their sequestration into CCVs. Presumably, CCV assembly occurs through the guanidine nucleotide exchange factor-mediated recruitment of ADP-ribosylation factor1 followed by adaptor protein complex1 (AP-1) adaptors, which attach to phospholipids and to the cytosolic tails of the VSRs (Park et al., 2013). (4) It is well known that the cation-dependent MPR must be present as a dimer in order to bind ligands (Olson et al., 2010). The same requirement exists for VSRs, and while VSR mutants incapable of oligomerizing may enter the Golgi apparatus, they proceed no farther and do not interact with clathrin (Kim et al., 2010). Thus, it can be expected that the ER is also the location where oligomerization (possibly trimerization, since the complex has a molecular mass of 240 kD and the molecular mass of VSRs in SDS-PAGE is around 80 kD) of VSRs takes place.
THE SEGREGATION OF SECRETORY AND VACUOLAR TRAFFIC AT THE TGN: POSSIBLE MECHANISMS
The TGN is a transport hub for incoming endocytic cargo and outgoing secretory and vacuolar cargo (Uemura and Nakano, 2013). It is continually formed as a consequence of cisternal progression through the Golgi stack and remains associated with the stack for a while before being detached (Viotti et al., 2010; Uemura et al., 2014). The TGN is a cisternal-tubular network bearing two morphologically different types of vesicle: smooth-surfaced secretory vesicles and CCVs. Traditionally, secretion is regarded as occurring by default, simply going with the flow, but recent work on mammalian and yeast cells (Curwin et al., 2012; von Blume et al., 2012) suggest that it is an active process involving a Ca2+-binding, secretory cargo-sequestering protein and a TGN-localized Ca2+ pump. The possibility that such a mechanism might operate in plants has been discussed (Robinson and Pimpl, 2014b).
It is also unclear whether there are functionally two different types of CCV at the plant TGN or only one. Since the TGN in plants probably acts as a recycling endosome as well as an EE, it is likely that one class of CCV serves to recycle internalized membrane proteins/receptors back to the PM. If there is another class of CCV, does it transport VSR-ligand complexes out of the Golgi, or is this achieved passively, as a result of cisternal release from the Golgi stack and subsequent maturation/transformation into a multivesicular PVC (Niemes et al., 2010b)? These two scenarios are depicted in Figure 2.
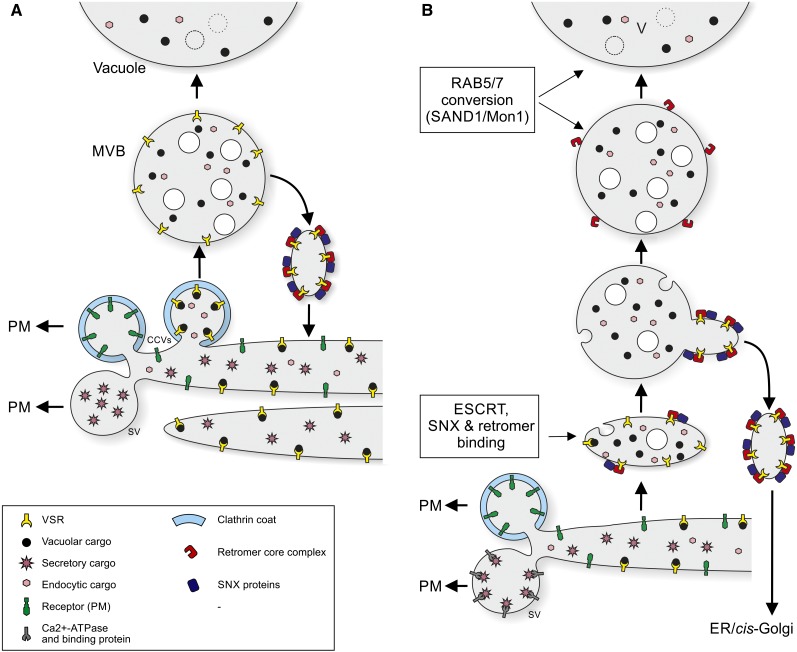
Figure 2.
Options for the sorting of soluble secretory and vacuolar cargo molecules at the TGN, together with possible models for post-Golgi recycling of VSRs. In option A, there are two classes of CCV formed at the TGN: one for recycling membrane proteins/receptors back to the PM after they have been internalized and transported to the EE(TGN) and the other serving to package VSR-ligand complexes for transport to the LE(MVB) and LE(PVC). Ligands dissociate from the VSRs in the MVBs, and retromer-coated carriers transport the VSRs back to the TGN. Secretory proteins are passively sorted into secretory vesicles at the TGN. This option is favored by Hwang (2008), De Marcos Lousa et al. (2012), Kang et al. (2012), and Xiang et al. (2013). In option B, it is proposed that, in analogy to mammalian and yeast cells, secretory proteins are actively sequestered into secretory vesicles via Ca2+-binding proteins/Ca2+-ATPase. In contrast, VSR-ligand complexes passively leave the Golgi stack through maturation/transformation of the TGN, which is released from the stack. As maturation proceeds, VSR-ligand dissociation takes place and the VSRs become concentrated in retromer carriers for transport back to the ER/cis-Golgi. In this model, there is only one type of CCV that is responsible for recycling membrane proteins back to the PM. Evidence for this option has been given by Niemes et al. (2010a, 2010b) and Robinson and Pimpl (2014a, 2014b).
If a transformation of the TGN into a multivesicular body (MVB) does occur, it is extremely difficult to obtain electron micrographs of intermediate stages depicting this process, suggesting that the maturation of the TGN into an MVB must be rapid. However, immunolabeling has demonstrated the presence of endosomal sorting complex required for transport (ESCRT) I and ESCRT II complex proteins (required for the initiation of internal vesicle formation in MVBs; Hurley and Hanson, 2010) at the TGN. In contrast, ESCRT III (required for the fission of the internal vesicles) was detected at the multivesicular PVC (Scheuring et al., 2011; Cai et al., 2014). Further evidence comes from the observation that some proteins that have previously been regarded as being markers for the MVB now also have been detected on discrete domains of the TGN (e.g. the Rab5 GTPase Arabidopsis Rab5GTPase-like protein7 and phosphatidylinositol 3-phosphate; Singh et al., 2014). Thus, there is increasing support for the notion that multivesicular PVCs are derived from the TGN through maturation in a similar manner to how late endosomes (LEs) mature out of EEs in mammalian cells (van Weering et al., 2010).
Evidence in favor of CCVs being the means of transporting soluble vacuolar cargo out of the TGN lies in the interaction of the cytosolic tails of VSRs with AP-1 adaptor proteins required for CCV assembly at the TGN (De Marcos Lousa et al., 2012; Gershlick et al., 2014). This appears to be a convincing argument, except that VSRs have also been detected at the PM and the Tyr motif in the VSR tail may instead be required for endocytosis (Saint-Jean et al., 2010). Moreover, the expression of clathrin hubs, which titrate out the clathrin light chains required for triskelion assembly and thereby prevent CCV formation (and endocytosis), does not inhibit vacuolar protein transport (Scheuring et al., 2011). Perhaps the most serious argument against CCV transporting VSR-ligand complexes out of the TGN is that it does not seem to make much sense to do this if the MVB with which the CCVs are supposed to fuse is also derived from the TGN. Unfortunately, studies on AP-1 adaptor mutants (Park et al., 2013; Robinson and Pimpl, 2014a), which might have delivered a decisive answer on this issue, have proved equivocal, since the expression of these mutants also had adverse effects on secretion and TGN functioning in general.
VSR RECYCLING: FROM WHERE?
Theoretically, VSRs should recycle from the compartment where ligands dissociate, and dissociation is supposed to be pH dependent (Martinière et al., 2013). Determinations of the pH for ligand binding to MPRs (Tong and Kornfeld, 1989) and VSRs (Kirsch et al., 1994) reveal bell-shaped curves with optima at around 6, decreasing to less than 50% at pH 5.5; in the case of the cation-dependent MPR, there is clear crystallographic evidence that low pH affects the protonation state of the ligand-binding pocket as well as the dimer conformation (Olson et al., 2008). There is thus good reason to expect VSRs to dissociate from their ligands at acidic pH. However, the expectation that multivesicular PVCs would fulfill this criterion has not been met. In fact, recent measurements of pH in the TGN and PVC point to an alkaline rather than an acid gradient between these two organelles (Martinière et al., 2013). So, for the moment, organelle pH is not a good indicator for the location of VSR-ligand dissociation.
A pentameric cytosolic coat complex called the retromer is responsible for the recycling of MPRs in mammals and VSRs in yeast (Attar and Cullen, 2010). The vacuolar protein sorting35 homolog (VPS35) subunit of the trimeric core retromer subcomplex has been shown to bind to the cytosolic tail of the MPR and to the VSR BP80 of plants (Oliviusson et al., 2006). The other subunit consists of the two sorting nexins (SNXs), which have PX domains for binding to phosphatidylinositol phosphates and BAR domains causing the membranes to tubularize (van Weering et al., 2010). The subcellular location of plant SNXs has been a matter of some debate (Robinson et al., 2012), with current evidence now favoring the TGN rather than multivesicular PVCs (Stierhof et al., 2013; Ivanov et al., 2014). Attempts to localize the retromer core subunit by immunogold electron microscopy and immunofluorescence microscopy have been performed, and in both cases the published data are contradictory (compare Oliviusson et al. [2006] with Niemes et al. [2010b]). However, mutants of the VPS35A and VPS29 retromer core subunits show defects in PIN-formed1-GFP transport and appear to have an altered PVC morphology (Nodzynski et al., 2013). Whether this reflects a retromer localization at the PVC, or is a consequence of retromer malfunction upstream of the PVC, is unclear.
In mammalian cells, maturing endosomes are characterized by tubular protuberances that are enriched in SNX1, the retromer core, and MPRs (Mari et al., 2008). These then pinch off to form torpedo-like retrograde transport carriers (Collins et al., 2008). The onset of tubule formation on the EE in mammals is coordinated with a transition from Rab5- to Rab7-type GTPases, culminating in the binding of the core retromer subunit (van Weering et al., 2012). Also required for the recruitment of the retromer core onto mammalian endosome membranes is SNX3, lacking BAR domains (Seaman, 2012). Surprisingly, in plants, Rab7 GTPases are found on the tonoplast and prefusion late PVCs (LPVCs; Nielsen et al., 2008; Bottanelli et al., 2012), whereas Rab5 GTPases are present on MVB/PVCs and not the TGN (Contento and Bassham, 2012). This was recently confirmed in investigations of SAND/Mon1 (for a protein family containing Sp100, AIRE-1, DEAF-1; with monensin sensitivity), the guanidine nucleotide exchange factor for Rab7 that also locates to LPVCs and the tonoplast (Cui et al., 2014; Ebine et al., 2014; Singh et al., 2014). Thus, and in marked contrast to the situation in other organisms, the Rab5-to-Rab7 conversion in plants is not associated with the maturation from EE(TGN) to LE(MVBs) but, instead, is required for MVB/LPVC-vacuole fusion. Mutants of both Rab7 and SAND/Mon1 show enlarged MVBs, and soluble vacuolar proteins are secreted (Ebine et al., 2014; Singh et al., 2014). Conversely, PIN cycling and the accumulation of the cytokinesis syntaxin KNOLLE at the cell plate were unaffected in SAND/Mon1 mutants, as was the localization of the TGN marker vacuolar protein ATPase A1, indicating that a Rab5-to-Rab7 conversion is not required for TGN-based secretory events (Singh et al., 2014).
Based on the data just described, the core subunit of plant retromer should also localize to the membranes of prefusion MVBs and the tonoplast, but this is difficult to reconcile with the fact that mature MVBs, even when optimally freeze fixed for electron microscopy, do not possess tubular extensions, and vesiculation profiles are extremely rare (Stierhof et al., 2013). Moreover, it has been shown that the retromer core subunit by itself is incapable of inducing endosomal membranes from mammalian cells to tubularize in vitro (van Weering et al., 2012). So, even though it has been demonstrated that Ras-related protein in brain G3f, GTPase is required for the binding of the retromer core to plant membranes (Zelazny et al., 2013), without the participation of SNXs, which appear to localize predominantly to the TGN (see above), it is difficult to understand how retromer can retrieve VSRs from the LPVC/tonoplast. In this regard, it is important to note that Foresti et al. (2010) characterized their LPVC as lacking VSRs. This suggests that retromer-mediated retrieval of VSRs must occur earlier than the Rab5-to-Rab7 conversion. This, and the demonstration that there are multiple pathways to the vacuole, some requiring a sequential Rab5-to-Rab7 transition and others needing only one of these GTPases (Ebine et al., 2014), underline once again the dangers of trying to dovetail plant data into a mammalian (yeast) template.
VSR RETRIEVAL: WHERE TO?
The target compartment for the recycling of VSRs has also become a subject for debate. The widely held opinion that VSRs are recycled to the TGN was challenged by Niemes et al. (2010b), who, based on their contention that VSR-ligand binding is initiated at the ER, suggested that, after ligand dissociation, VSRs also must be delivered to this location to start a new cycle of anterograde transport of cargo ligands. Since the new data of Gershlick et al. (2014) support the occurrence of VSR-ligand interactions very early in the secretory pathway, I would have expected agreement on the matter of VSR recycling, but Gershlick et al. (2014) have stated, “It is plausible that VSRs return directly to the trans-Golgi cisternae, as it would explain why VSRs can be enriched in this compartment (Hillmer et al., 2001), but this remains to be shown.” It is difficult to follow the logic behind this conclusion. What function would recycled free VSRs have in the TGN if there are no free ligands, since vacuolar cargo ligands are already bound to VSRs upon entry to the TGN? Only if VSR-ligand binding at the ER or cis-Golgi were inefficient, leading to high concentrations of unbound ligands at the TGN, would this make sense.
Obviously, the oligomerization status of recycled VSRs will be crucial: if upon ligand dissociation they assume a monomeric form, they would be nonfunctional when reinserted into the TGN. However, this would not matter if recycling to the ER takes place where, as discussed earlier, oligomerization presumably takes place. In this regard, the situation in mammalian cells is worth bearing in mind. Although phosphomannans are first available in the TGN, the small 46-kD MPR nevertheless dimerizes and attains its ligand-binding ability before exiting the ER (Hille et al., 1990). However, as determined by in situ cross-linking studies, the dimer is the dominant form of the 46-kD MPR in both the TGN and the EE (Punnonen et al., 1996). Thus, although a pH-dependent change in MPR conformation may take place upon entry into the EE, resulting in ligand dissociation, it is not accompanied by a change in oligomeric status.
Clearly, there are many evolutionarily conserved aspects of the secretory and endocytic pathways in eukaryotes, but, as illuminated in this article, there are an equal number of significant differences between the major organismal groups. So, in conclusion, great care is needed when a well-established scheme of events in animal cells is used as a basis for the interpretation of plant data.
Acknowledgments
I thank Dr. Peter Pimpl (Centre for Molecular Biology of Plants, Tuebingen) for useful discussions and for preparing the cartoon figures.
Glossary
- MPR
mannosyl 6-phosphate receptor
- TGN
trans-Golgi network
- CCV
clathrin-coated vesicle
- EE
early endosome
- VSR
vacuolar sorting receptor
- PVC
prevacuolar compartment
- ER
endoplasmic reticulum
- LBD
ligand-binding domain
- PM
plasma membrane
- MVB
multivesicular body
- LPVC
late prevacuolar compartment
References
- Attar N, Cullen PJ. (2010) The retromer complex. Adv Enzyme Regul 50: 216–236 [DOI] [PubMed] [Google Scholar]
- Bottanelli F, Gershlick DC, Denecke J. (2012) Evidence for sequential action of Rab5 and Rab7 GTPases in prevacuolar organelle partitioning. Traffic 13: 338–354 [DOI] [PubMed] [Google Scholar]
- Braulke T, Bonifacino JS. (2009) Sorting of lysosomal proteins. Biochim Biophys Acta 1793: 605–614 [DOI] [PubMed] [Google Scholar]
- Cai Y, Zhuang X, Gao C, Wang X, Jiang L. (2014) The Arabidopsis endosomal sorting complex required for transport III regulates internal vesicle formation of prevacuolar compartment and is required for plant development. Plant Physiol 165: 1328–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, Norwood SJ, Kerr MC, Mahony D, Seaman MN, Teasdale RD, Owen DJ. (2008) Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic 9: 366–379 [DOI] [PubMed] [Google Scholar]
- Contento AL, Bassham DC. (2012) Structure and function of endosomes in plant cells. J Cell Sci 125: 3511–3518 [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhao Q, Gao C, Ding Y, Zeng Y, Ueda T, Nakano A, Jiang L. (2014) Activation of the Rab7 GTPase by the MON1-CCZ1 complex is essential for PVC-to-vacuole trafficking and plant growth in Arabidopsis. Plant Cell 26: 2080–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin AJ, von Blume J, Malhotra V. (2012) Cofilin-mediated sorting and export of specific cargo from the Golgi apparatus in yeast. Mol Biol Cell 23: 2327–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J. (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17: 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcos Lousa C, Gershlick DC, Denecke J. (2012) Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors. Plant Cell 24: 1714–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebine K, Inoue T, Ito J, Ito E, Uemura T, Goh T, Abe H, Sato K, Nakano A, Ueda T. (2014) Plant vacuolar trafficking occurs through distinctly regulated pathways. Curr Biol 24: 1375–1382 [DOI] [PubMed] [Google Scholar]
- Foresti O, Gershlick DC, Bottanelli F, Hummel E, Hawes C, Denecke J. (2010) A recycling-defective vacuolar sorting receptor reveals an intermediate compartment situated between prevacuoles and vacuoles in tobacco. Plant Cell 22: 3992–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershlick DC, de Marcos Lousa C, Foresti O, Lee AJ, Pereira EA, daSilva LL, Bottanelli F, Denecke J. (2014) Golgi-dependent transport of vacuolar sorting receptors is regulated by COPII, AP1, and AP4 protein complexes in tobacco. Plant Cell 26: 1308–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille A, Waheed A, von Figura K. (1990) Assembly of the ligand-binding conformation of Mr 46,000 mannose 6-phosphate-specific receptor takes place before reaching the Golgi complex. J Cell Biol 110: 963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer S, Movafeghi A, Robinson DG, Hinz G. (2001) Vacuolar storage proteins are sorted in the cis-cisternae of the pea cotyledon Golgi apparatus. J Cell Biol 152: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G, Colanesi S, Hillmer S, Rogers JC, Robinson DG. (2007) Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic 8: 1452–1464 [DOI] [PubMed] [Google Scholar]
- Hurley JH, Hanson PI. (2010) Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol 11: 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I. (2008) Sorting and anterograde trafficking at the Golgi apparatus. Plant Physiol 148: 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov R, Brumbarova T, Blum A, Jantke AM, Fink-Straube C, Bauer P. (2014) SORTING NEXIN1 is required for modulating the trafficking and stability of the Arabidopsis IRON-REGULATED TRANSPORTER1. Plant Cell 26: 1294–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Kim SY, Song K, Sohn EJ, Lee Y, Lee DW, Hara-Nishimura I, Hwang I. (2012) Trafficking of vacuolar proteins: the crucial role of Arabidopsis vacuolar protein sorting 29 in recycling vacuolar sorting receptor. Plant Cell 24: 5058–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kang H, Jang M, Chang JH, Miao Y, Jiang L, Hwang I. (2010) Homomeric interaction of AtVSR1 is essential for its function as a vacuolar sorting receptor. Plant Physiol 154: 134–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Paris N, Butler JM, Beevers L, Rogers JC. (1994) Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc Natl Acad Sci USA 91: 3403–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Bujny MV, Zeuschner D, Geerts WJ, Griffith J, Petersen CM, Cullen PJ, Klumperman J, Geuze HJ. (2008) SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 9: 380–393 [DOI] [PubMed] [Google Scholar]
- Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N. (2013) In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 25: 4028–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J, Paris N. (2005) Plant vacuoles: from biogenesis to function. Plant Cell Monogr 1: 63–82 [Google Scholar]
- Nielsen E, Cheung AY, Ueda T. (2008) The regulatory RAB and ARF GTPases for vesicular trafficking. Plant Physiol 147: 1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemes S, Labs M, Scheuring D, Krueger F, Langhans M, Jesenofsky B, Robinson DG, Pimpl P. (2010a) Sorting of plant vacuolar proteins is initiated in the ER. Plant J 62: 601–614 [DOI] [PubMed] [Google Scholar]
- Niemes S, Langhans M, Viotti C, Scheuring D, San Wan Yan M, Jiang L, Hillmer S, Robinson DG, Pimpl P. (2010b) Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J 61: 107–121 [DOI] [PubMed] [Google Scholar]
- Nodzynski T, Feraru MI, Hirsch S, De Rycke R, Niculaes C, Boerjan W, Van Leene J, De Jaeger G, Vanneste S, Friml J. (2013) Retromer subunits VPS35A and VPS29 mediate prevacuolar compartment (PVC) function in Arabidopsis. Mol Plant 6: 1849–1862 [DOI] [PubMed] [Google Scholar]
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG. (2006) Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LJ, Hindsgaul O, Dahms NM, Kim JJ. (2008) Structural insights into the mechanism of pH-dependent ligand binding and release by the cation-dependent mannose 6-phosphate receptor. J Biol Chem 283: 10124–10134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LJ, Sun G, Bohnsack RN, Peterson FC, Dahms NM, Kim JJ. (2010) Intermonomer interactions are essential for lysosomal enzyme binding by the cation-dependent mannose 6-phosphate receptor. Biochemistry 49: 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordenes VR, Moreno I, Maturana D, Norambuena L, Trewavas AJ, Orellana A. (2012) In vivo analysis of the calcium signature in the plant Golgi apparatus reveals unique dynamics. Cell Calcium 52: 397–404 [DOI] [PubMed] [Google Scholar]
- Park M, Song K, Reichardt I, Kim H, Mayer U, Stierhof YD, Hwang I, Jürgens G. (2013) Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc Natl Acad Sci USA 110: 10318–10323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson BA, Pimpl P, daSilva LL, Crofts AJ, Taylor JP, Movafeghi A, Robinson DG, Denecke J. (2001) Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13: 2005–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen EL, Wilke T, von Figura K, Hille-Rehfeld A. (1996) The oligomeric state of 46-kDa mannose 6-phosphate receptor does not change upon intracellular recycling and binding of ligands. Eur J Biochem 237: 809–818 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Pimpl P. (2014a) Clathrin and post-Golgi trafficking: a very complicated issue. Trends Plant Sci 19: 134–139 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Pimpl P. (2014b) Receptor-mediated transport of vacuolar proteins: a critical analysis and a new model. Protoplasma 251: 247–264 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Pimpl P, Scheuring D, Stierhof YD, Sturm S, Viotti C. (2012) Trying to make sense of retromer. Trends Plant Sci 17: 431–439 [DOI] [PubMed] [Google Scholar]
- Saint-Jean B, Seveno-Carpentier E, Alcon C, Neuhaus JM, Paris N. (2010) The cytosolic tail dipeptide Ile-Met of the pea receptor BP80 is required for recycling from the prevacuole and for endocytosis. Plant Cell 22: 2825–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Delgadillo MO, Zouhar J, Reynolds GD, Pennington JG, Jiang L, Liljegren SJ, Stierhof YD, De Jaeger G, Otegui MS, et al. (2013) MTV1 and MTV4 encode plant-specific ENTH and ARF GAP proteins that mediate clathrin-dependent trafficking of vacuolar cargo from the trans-Golgi network. Plant Cell 25: 2217–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuring D, Viotti C, Krüger F, Künzl F, Sturm S, Bubeck J, Hillmer S, Frigerio L, Robinson DG, Pimpl P, et al. (2011) Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23: 3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN. (2012) The retromer complex: endosomal protein recycling and beyond. J Cell Sci 125: 4693–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Krüger F, Beckmann H, Brumm S, Vermeer JEM, Munnik T, Mayer U, Stierhof YD, Grefen C, Schumacher K, et al. (2014) Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr Biol 24: 1383–1389 [DOI] [PubMed] [Google Scholar]
- Stierhof YD, Viotti C, Scheuring D, Sturm S, Robinson DG. (2013) Sorting nexins 1 and 2a locate mainly to the TGN. Protoplasma 250: 235–240 [DOI] [PubMed] [Google Scholar]
- Tong PY, Kornfeld S. (1989) Ligand interactions of the cation-dependent mannose 6-phosphate receptor: comparison with the cation-independent mannose 6-phosphate receptor. J Biol Chem 264: 7970–7975 [PubMed] [Google Scholar]
- Uemura T, Nakano A. (2013) Plant TGNs: dynamics and physiological functions. Histochem Cell Biol 140: 341–345 [DOI] [PubMed] [Google Scholar]
- Uemura T, Suda Y, Ueda T, Nakano A. (2014) Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol 55: 694–703 [DOI] [PubMed] [Google Scholar]
- van Weering JR, Sessions RB, Traer CJ, Kloer DP, Bhatia VK, Stamou D, Carlsson SR, Hurley JH, Cullen PJ. (2012) Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J 31: 4466–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Verkade P, Cullen PJ. (2010) SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin Cell Dev Biol 21: 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, et al. (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Blume J, Alleaume AM, Kienzle C, Carreras-Sureda A, Valverde M, Malhotra V. (2012) Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J Cell Biol 199: 1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E, Shimada T, Kuroyanagi M, Nishimura M, Hara-Nishimura I. (2002) Calcium-mediated association of a putative vacuolar sorting receptor PV72 with a propeptide of 2S albumin. J Biol Chem 277: 8708–8715 [DOI] [PubMed] [Google Scholar]
- Watanabe E, Shimada T, Tamura K, Matsushima R, Koumoto Y, Nishimura M, Hara-Nishimura I. (2004) An ER-localized form of PV72, a seed-specific vacuolar sorting receptor, interferes the transport of an NPIR-containing proteinase in Arabidopsis leaves. Plant Cell Physiol 45: 9–17 [DOI] [PubMed] [Google Scholar]
- Xiang L, Etxeberria E, Van den Ende W. (2013) Vacuolar protein sorting mechanisms in plants. FEBS J 280: 979–993 [DOI] [PubMed] [Google Scholar]
- Zelazny E, Santambrogio M, Pourcher M, Chambrier P, Berne-Dedieu A, Fobis-Loisy I, Miège C, Jaillais Y, Gaude T. (2013) Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis. J Biol Chem 288: 8815–8825 [DOI] [PMC free article] [PubMed] [Google Scholar]