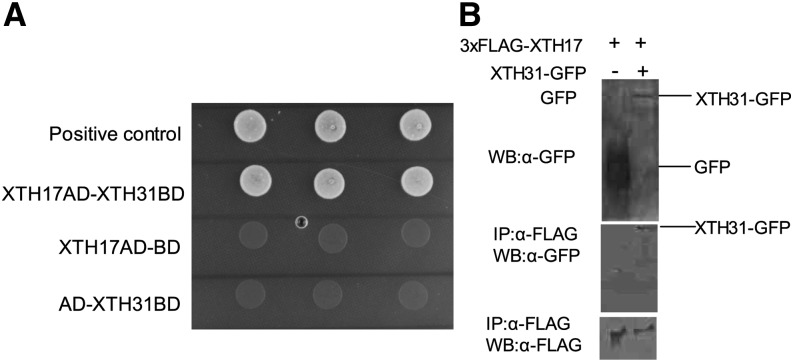
Figure 1.
Physical interaction between XTH17 and XTH31. A, Yeast two-hybrid analysis of XTH17 and XTH31. XTH31 (with DNA-binding domain [BD]) and XTH17 (with activation domain [AD]) were introduced into yeast cells as indicated. Colony growth indicates binding domain and activation domain proximity enabled through interaction of proteins. The empty vector of binding domain with XTH17-AD and the empty vector of activation domain with XTH31-BD are represented as negative control. Three colonies for each line means replicates. B, Coimmunoprecipitation of 3XFlag-XTH17 and XTH31-GFP using tobacco. Total protein extracts (top) or proteins immunoprecipitated (IP) using anti-GFP (α-GFP) antibody from the solubilized microsomal and cytosolic fractions (middle and bottom) were analyzed by western blotting (WB) using α-GFP or α-FLAG antibodies.