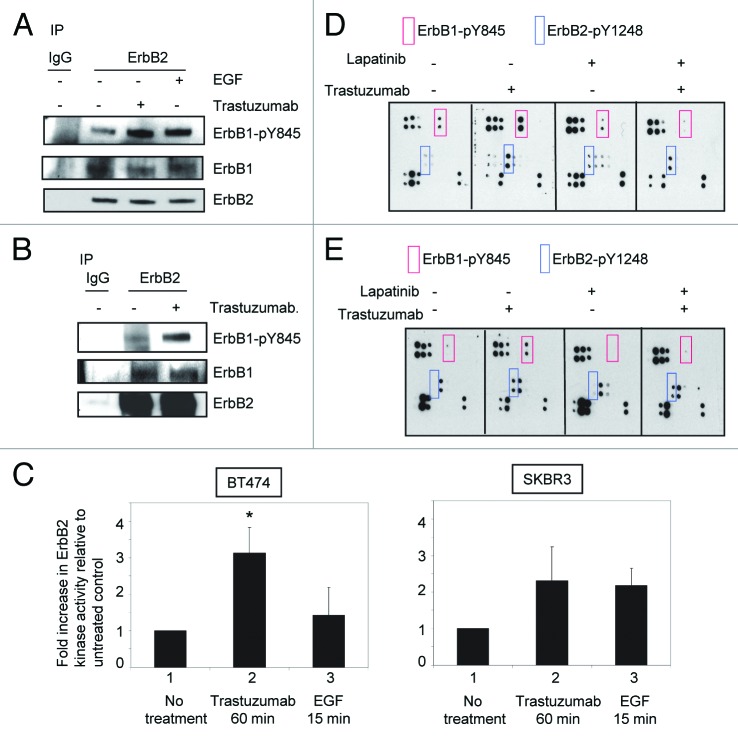
Figure 2. Trastuzumab activates ErbB2 tyrosine kinase and induces ErbB2-Y1248 phosphorylation in the presence of lapatinib. (A) Increased ErbB1-Y845 phosphorylation was detected following trastuzumab treatment of SKBR3 cells. Serum-starved SKBR3 cells were treated for 1 h. After harvesting the WCL, the immunoprecipitation reaction with antibody recognizing ErbB2 (29D8) was performed to detect the heterodimer between ErbB1 and ErbB2. (B) The experiments were performed similar to those described in (A) except that prior to harvesting the WCL, trastuzumab-treated BT474 cells were crosslinked with DTSSP reagent according to the modified protocol provided in the literature.5 (C) Trastuzumab induces the activation of ErbB2 kinase activity in BT474 and SKBR3 cells. After serum-starving overnight, SKBR3 and BT474 cells were either treated with trastuzumab for 1 h or EGF for 15 min, or left untreated as indicated. WCL were harvested and subjected to immunoprecipitation using either human control IgG or trastuzumab. ErbB2 tyrosine kinase activity in each immunoprecipitate was determined using a universal tyrosine kinase assay kit (Takara Bio Inc.) according to manufacturer’s instructions. Data are expressed as mean ± SEM. Statistical significance was determined by the Student t test. *P < 0.05. (D) SKBR3 cells were plated and grown in the serum-containing media and then serum-starved overnight. Cells were either pre-treated with lapatinib (200 nM) for 4 h or not pretreated and then either treated with trastuzumab (4 μg/mL) or left untreated. Analysis of phosphorylation levels of ErbB family phosphorylation sites was done by RayBio human EGFR phosphorylation antibody array 1 according to the manufacturer’s instructions. (E) Analysis of phosphorylation level in ErbB family receptors following treatment of BT474 cells with trastuzumab, lapatinib, or trastuzumab plus lapatinib. The experimental procedures were essentially the same as those described in (D).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.