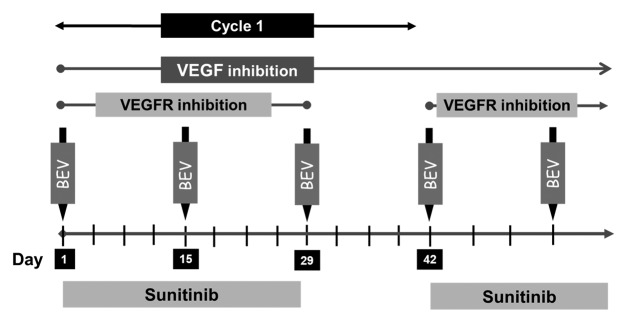
Figure 2. Study schema: Patients received sunitinib 37.5 mg PO daily from weeks 1–4 and bevacizumab (Bev) 5 mg/kg intravenously on days 1, 15, and 29 of each 6-wk cycle.

Figure 2. Study schema: Patients received sunitinib 37.5 mg PO daily from weeks 1–4 and bevacizumab (Bev) 5 mg/kg intravenously on days 1, 15, and 29 of each 6-wk cycle.