Abstract
Glucocorticoids are effective drugs used for the treatment of inflammatory diseases such as rheumatoid arthritis or asthma. Furthermore, they regulate various physiological processes, including bone remodeling. However, long-term high- and even low-dose glucocorticoid use is associated with a compromised bone quality and an increased fracture risk. At the cellular level, glucocorticoids suppress bone formation and stimulate bone resorption, which leads to loss of bone mass. To investigate the underlying mechanisms and new therapeutic strategies, the in vivo model for glucocorticoid-induced bone loss is widely used. This protocol outlines the common procedure that is currently used for the induction of bone loss in mice using glucocorticoids. It further provides useful hints and highlights possible pitfalls to take into account before starting an experiment.
Introduction
Bone loss induced by long-term glucocorticoid intake is a frequent complication of steroid treatment and the underlying mechanisms have not been fully elucidated yet. A common model for simulating chronic glucocorticoid use is treating mice with slow-release pellets containing prednisolone. The formulation of the slow-release pellets in the current protocol allows a constant release of prednisolone over a 4-week period, and thus mimics the chronic exposure of patients to glucocorticoids.
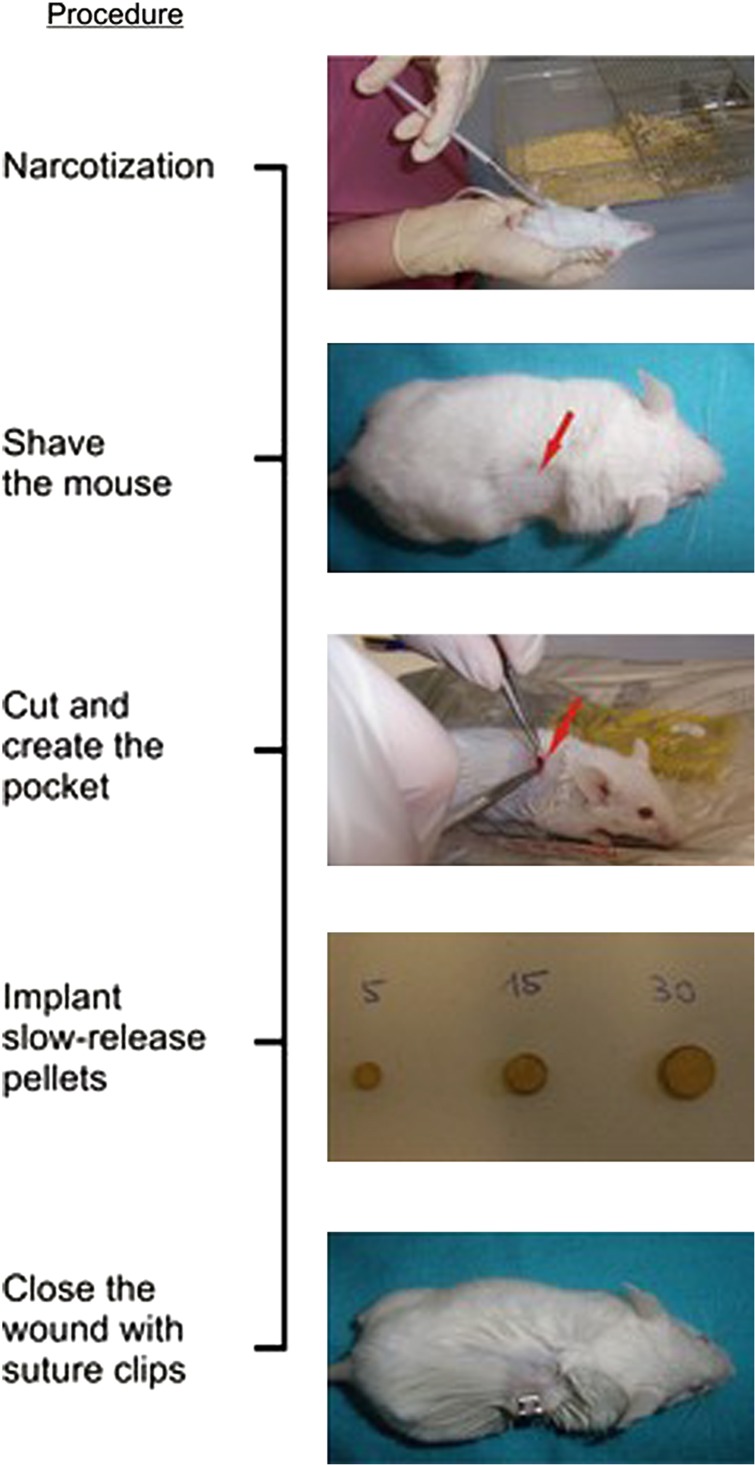
Briefly, the procedure works as follows: take 24-week-old female mice and implant 60-day slow-release pellets (see Figure 1) containing placebo or prednisolone subcutaneously. Check on mice weekly to make sure the wound is healing properly. After a total of 4 weeks, kill the mice to examine the effects on the skeleton.1,2,3
Figure 1.
Procedure for the induction of glucocorticoid-induced bone loss in FVB/N mice.
Materials and methods
Materials
 |
Methods
Considerations before starting the experiment.
Gender: Bone loss by glucocorticoids can be induced in both genders. An advantage of using male mice is to avoid possible influences of the female endocrine system. However, because of the ranking order, male mice commonly have to be separated into smaller groups or even be isolated (Table 1).
Table 1. Overview of different models for glucocorticoid-induced bone loss given in the reported dose.
| Strain | Gender | Age (weeks) | Pellet dose (mg)a | Doseb (mg per kg BW per d) | Duration (weeks) | Source |
|---|---|---|---|---|---|---|
| FVB/N | Female | 24 | 3.5 | 4 | 3 | |
| Intercross: Black Swiss and 129SvJ | Male | 32 | 5 | 2.1 | 4 | 1,4 |
| Swiss Webster | Male | 24 | 5 | 0–8 | 5 | |
| Swiss Webster | Male | 28 | 2.1 | 8 | 2 | |
| Swiss Webster | Male | 24 | 1.4 | 3 | 6 | |
| FVB/N, 129SvJ | Female | 10 | 15 | 12.5 | 2 | 7 |
| C57BL/6 | Male | 20 | 7.5 | 4 | 8 |
Abbreviations: BW, body weight; d, day.
aUsing 60-day slow-release pellets
bFor scaling the dose per mouse to the equivalent dose in humans, the dose per kg BW should be calculated with the weight in relation to the metabolic rate (for mice, weight in kg to the ¾ power9).
Age: Take into account that the age is an important factor. Younger mice (12 weeks) do not lose bone mineral density after glucocorticoid exposure; therefore, mice with complete skeletal maturity (around 20 weeks) should be used (Table 1).
Strain: Not all mouse strains are susceptible to glucocorticoid-induced bone loss. A very susceptible strain for this model is the FVB/N mouse, but also Swiss Webster or C57BL/6 mice lose bone after long-term glucocorticoid exposure (Table 1).
Dose: Depending on the strain used, the glucocorticoid dose may need to be adjusted. C57BL/6 mice, for example, require a higher dose of glucocorticoids as compared with FVB/N mice. A total prednisolone dose of 3.5 mg for FVB/N and 7.5 mg for C57BL/6 mice corresponds to ∼4 mg per kg body weight per day and 9 mg per kg body weight per day, respectively, assuming a body weight of around 30 g. As prednisolone is well tolerable, higher doses can also be used to induce bone loss within a shorter period of time (for example, 15 mg pellets over 2 weeks; see Table 1).
Procedure (for the C57BL/6 mouse strain)
Order an appropriate number of 60-day slow-release pellets containing 7.5 mg prednisolone (or equivalent) for C57BL/6 mice. Order the same amount of placebo pellets as a control.
Wait until the mice are at least 24 weeks old to ensure the development of detectable signs of bone loss.
Anesthetize mice by intraperitoneal injection of a combination of ketamine (100 mg per kg body weight) and xylazine (20 mg per kg body weight). Note that other methods of anesthesia can also be applied (for example, isofluran).
Once the mice are sleeping, shave a small area above the scapula (20 × 20 mm; see Figure 1) and disinfect the area using Cutasept F.
Tweeze the skin and make a small cut (5 mm) using scissors (or a scalpel). Widen the cut by straddling the handles carefully to create a pocket (see Figure 1).
Place the pellet in the pocket under the skin by using the tweezers. Make sure the pellet is placed well inside the pocket and does not slip out to the surface again.
Clamp the wound with suture clips (see Figure 1).
Place the mouse back in the cage and check the next day whether the mouse is behaving normally and the wound is still closed.
Check the wound healing (inflammation possible) twice weekly and observe how the mice handle their wound clips, especially when they are together in groups. Carefully observe mouse behavior, which may change after glucocorticoid treatment.
Kill mice after 4 weeks for further analysis. Check whether the pellets are completely dissolved. Regardless of further analysis, it is advisable to check bone mineral density to confirm the success of the procedure.
NOTE: The bone loss induced by glucocorticoids in C57BL/6 mice is rather subtle (8–20%, depending on the skeletal site). Thus, make sure your sample size is adequate (usually 7–8 mice per group) to detect significant differences in bone mineral density. Moreover, assessing the suppression of bone formation is usually a good surrogate for determining the success of glucocorticoid treatment, which can be investigated using serum markers of bone formation or dynamic bone histomorphometry.
Discussion
This protocol provides information on the common experimental procedure currently used to induce bone loss in mice using glucocorticoids. Given the critical role of glucocorticoids in the treatment of inflammatory diseases, there is a demanding need to provide valuable insights into the development and progression of glucocorticoid-induced bone loss. Furthermore, this method allows for the evaluation of new treatment strategies in preclinical research. Despite these advantages, the influence of different mouse strains, gender, age and dose must be considered for planning such in vivo studies. As mentioned in this protocol, carefully designed experimental protocols can minimize these pitfalls and help achieve the desired outcomes of the study.
Recommended further reading
The following sources document methods and standard protocols for the induction of glucocorticoid-induced bone loss in more detail as well as further information regarding to osteoporosis, glucocorticoids, glucocorticoid-induced osteoporosis and histomorphometry.
Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007;18:1319-1328.
Hofbauer LC, Rauner M. Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol Endocrinol 2009;23:1525-1531.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377:1276-1287.
Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 2010;11:517-531.
Weinstein RS. Glucocorticoid-induced bone disease. N Engl J Med 2011;365:62-70.
Yao W, Cheng Z, Pham A, Busse C, Zimmermann EA, Ritchie RO, Lane NE. Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum 2008;58:3485-3497.
Footnotes
The authors declare no conflict of interest.
References
- Hofbauer LC, Zeitz U, Schoppet M, Skalicky M, Schuler C et al. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum 2009;60:1427–1437. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 1998;102:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele S, Ziegler N, Tsourdi E, De Bosscher K, Tuckermann JP et al. Selective glucocorticoid receptor modulation maintains bone mineral density in mice. J Bone Miner Res 2012;27:2242–2250. [DOI] [PubMed] [Google Scholar]
- Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U et al. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol 2009;175:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum 2008;58:1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NE, Yao W, Balooch M, Nalla RK, Balooch G et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res 2006;21:466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 2010;11:517–531. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC. Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J Biol Chem 2011;286:44326–44335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson IW, Parker JC, Beliles RP. Biological basis for extrapolation across mammalian species. Regul Toxicol Pharmacol 1986;6:211–237. [DOI] [PubMed] [Google Scholar]