Abstract
Exposure to psychological trauma (for example, childhood/early life adversity, exposure to violence or assault, combat exposure, accidents or natural disasters) is known to increase one's risk of developing certain chronic medical conditions. Clinical and population studies provide evidence of systemic inflammatory activity in trauma survivors with various psychiatric and nonpsychiatric conditions. This transdiagnostic meta-analysis quantitatively integrates the literature on the relationship of inflammatory biomarkers to trauma exposure and related symptomatology. We conducted random effects meta-analyses relating trauma exposure to log-transformed inflammatory biomarker concentrations, using meta-regression models to test the effects of study quality and psychiatric symptomatology on the inflammatory outcomes. Across k=36 independent samples and n=14 991 participants, trauma exposure was positively associated with C-reactive protein (CRP), interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (mean rs =0.2455, 0.3067, 0.2890, and 0.2998, respectively). No significant relationships were noted with fibrinogen, IL-2, IL-4, IL-8, or IL-10. In meta-regression models, the presence of psychiatric symptoms was a significant predictor of increased effect sizes for IL-1β and IL-6 (β=1.0175 and 0.3568, respectively), whereas study quality assessment scores were associated with increased effect sizes for IL-6 (β=0.3812). Positive correlations between inflammation and trauma exposure across a range of sample types and diagnoses were found. Although reviewed studies spanned an array of populations, research on any one specific psychiatric diagnosis was generally limited to one or two studies. The results suggest that chronic inflammation likely represents one potential mechanism underlying risk of health problems in trauma survivors.
Introduction
Chronic inflammation may be a hallmark of many chronic diseases, including cardiovascular disease (CVD), diabetes, and chronic pain disorders, among others. A history of exposure to traumatic events (for example, early life adversity, exposure to violence or assault, combat exposure, accidents, or natural disasters) is known to increase one's risk of developing chronic medical problems,1,2 and research on inflammatory biomarkers has begun to elucidate some of the potential mechanisms underlying this increased risk.
Physiological mechanisms linking the experience of psychological stressors to immune functioning are complex, with influences exerted through various pathways. Briefly, in response to a traumatic stressor, the biological stress systems (including sympathetic/parasympathetic, catecholamine, hypothalamic-pituitary-adrenal axis, and immune system components) assist in promoting adaptive behavioral and physiological responses to the stressor.3, 4, 5 Severe, repeated and prolonged exposure to traumatic stressors, however, can lead to chronic dysregulation of these basic biological systems. Chronic, systemic inflammation has been posited as one mechanism underlying psychiatric symptomatology, across a range of disorders,6, 7, 8 as well as with increased risk of many physical health problems.2,9,10
Historically, research into the physiological mechanisms occurring within the context of psychopathology has been segregated according to diagnostic classification. However, despite differing symptom presentations, mounting evidence of neurobiological, genetic, and physiological mechanisms underlying a range of physical and psychological disorders has led to increasing recognition that current diagnostic classifications may no longer provide an adequate framework for psychobiological research or for translating this research into clinical practice.11,12 This awareness has led to transdiagnostic initiatives such as the US National Institutes of Mental Health (NIMH) Research Domain Criteria project.13 Indeed, studies of immune activity across multiple psychiatric disorders, including posttraumatic stress disorder (PTSD),5 major depression,14 and bipolar disorder15,16 have all identified disruptions in proinflammatory cytokines (such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β), among symptomatic individuals, as compared with healthy control participants. In addition, a convergence of evidence has identified lifetime trauma exposure, particularly early life adversity, as a major risk factor for a range of chronic physical and psychiatric conditions,1,2 and systemic inflammation has been suggested as one potential mechanism mediating this association.2,4,5,17
Despite a rapidly growing body of literature on the relationships between trauma exposure and inflammatory biomarkers (including cytokines and acute-phase proteins, such as C-reactive protein (CRP) and fibrinogen) in both clinical and nonclinical samples, few attempts have been made to quantitatively integrate this research. To our knowledge, all existing meta-analyses have been limited to disorder-specific comparisons of symptomatic vs asymptomatic individuals, such as in depressive disorders14,18, 19, 20, 21 or bipolar disorder.15,16 In addition to the narrow focus on specific psychiatric diagnoses, prior meta-analyses have faced methodological challenges, including skewed biomarker concentrations in primary studies, disparate statistical techniques used to evaluate data, and systematic exclusion of large, population-based regression studies, which are often better equipped to statistically control for covariates, such as body mass index (BMI), age, sex, and the use of medications or other substances, all of which have previously been identified as important in biobehavioral research.22 Using meta-analysis and meta-regression models, the present study therefore describes the relationships between trauma history and in vivo inflammatory biomarkers (that is, cytokines and acute-phase proteins) from a transdiagnostic perspective.
Materials and Methods
Protocol
This study adhered to PRISMA guidelines for meta-analysis.23 Search strategy and data extraction were informed by a preliminary review of the literature and further specified on the basis of data availability and methodological variation, including limiting the present analysis to unstimulated in vivo cytokines and acute-phase proteins.
Inclusion/exclusion criteria
To address the relevant transdiagnostic theoretical questions, we included all studies of adult participants that analyzed unstimulated, in vivo (circulating) inflammatory biomarkers in blood samples (that is, cytokines, CRP, or fibrinogen) in relation to measures of trauma exposure. We excluded child and adolescent samples due to evidence that cytokine production in children differs substantially from that of adults, even among healthy populations.24,25 Trauma exposure was defined either through self-report measures of trauma or abuse (for example, Adverse Childhood Experiences questionnaire26) or by exposure to events meeting criterion A for PTSD.27 Studies that only included pre-trauma cytokine measurements were excluded. The statistical analyses assessed the impact of trauma exposure, and meta-regression models assessed the impact of relevant covariates, including study quality and the presence or absence of psychiatric symptomatology within the samples. As trauma exposure can be assessed either as a continuous or dichotomous variable, our inclusion criteria were intentionally inclusive of either design. Because only a small number of studies had adequate data to test the relative contribution of posttraumatic symptomatology within trauma-exposed populations (that is, comparing trauma-exposed symptomatic individuals to trauma-exposed controls), such analyses were underpowered and are therefore reported solely as supplemental analyses (see Supplementary Material). Thus, we chose to exclude studies that did not include either a continuous measure of trauma exposure or a non-trauma-exposed comparison group.
Study selection
Studies were identified through systematic searches of PubMed, PsycInfo, PILOTS (Published International Literature on Traumatic Stress), and Ovid MEDLINE databases. Search criteria included peer-reviewed articles published in English, using terms related to inflammation (for example, interleukin, cytokine) and either traumatic event exposure (for example, trauma* stress, child* maltreatment) or psychiatric conditions commonly linked to trauma exposure (for example, PTSD, depression), limiting the results to human samples. Additional selected references were identified through limited update searches, citations in other papers, and personal communications with authors (see Supplementary Material for full search strategy).
Study characteristics and data extraction
Data extraction
A single effect size estimate was calculated from each fully independent sample of participants for each biomarker. Potential duplicate publications were identified (within each biomarker outcome) through cross-checking authors' names and sample locations. Evaluation of inclusion/exclusion criteria and extraction of relevant data were conducted systematically by one of two coders, with a selection evaluated by both coders. Any disagreements (less than 5%) regarding eligibility or extracted data were settled by consensus.
Effect size and other study data were extracted from each of the published reports. Forms were piloted and revised as needed for extraction of relevant information. All assessments were made at the outcome level, with separate biomarkers analyzed independently. In the cases for which published data were insufficient, at least three attempts were made to contact authors. In total, the authors of 38 studies were contacted requesting information about sample independence, study eligibility or effect size calculation. Response rate was 76%, although some of these studies were not included in the present analyses (due to inclusion/exclusion criteria).
Data preparation and statistical analysis
Effect size preparation
A single effect size was calculated for each biomarker measured in an independent sample. Seven articles were confirmed by study authors to be duplicate samples, and one published report28 contained two independent subsamples (that is, African American and Caucasian participants), which were treated individually. Due to inclusion of both continuous and dichotomous predictors, we used correlation coefficients to synthesize the literature.29
As inflammatory biomarker distributions tend to be skewed, authors generally take one of three approaches to statistical testing and reporting of data: Use of nonparametric statistical methods, dichotomization according to clinically-relevant cut points (most frequently CRP>3 mg /L30), and logarithmic transformations to normalize the distributions before using parametric statistics.
Because data are log-transformed before statistical aggregation (individual data are converted into an exponent-scale before taking an arithmetic mean), effects based on log-transformed data cannot be mixed in the same analyses as raw (non-transformed) data. Log-transformed biomarker data are more likely to meet assumptions of normality than raw data; therefore, we chose to convert all raw effect sizes to estimated loge-transformed effect sizes,31 which were then converted to a log10 scale. When log-transformations were applied, any zero values were set to the smaller of the assay detection limit or a raw value of 1.0 (corresponding to a log-transformed value of 0), to allow for transformation and avoid artificially inflating the effect size estimate. Effect sizes for one study,32 for which we were unable to obtain effect size estimates by usual means, were extrapolated from a published scatterplot of individual participant data using WebPlotDigitizer, version 2.6 (http://arohatgi.info/WebPlotDigitizer/). Other effect size conversions were performed according to standard methods.29,33,34
Synthesis of results
We produced meta-analytic models for all analyses including at least three independent effect size estimates. Correlation coefficients were converted to Fisher's Z-values for analyses and back-converted to correlations for interpretation. All meta-analysis and meta-regression models were fitted using Wilson's meta-analysis macros for SPSS.35,36 Because we expected at least a moderate level of heterogeneity across studies, we used non-iterative method of moments random-effects models to integrate study findings. This approach produces wider confidence intervals and, thus, a more conservative estimate than either fixed- or other random-effects models.29 Forest plots were created,37 and heterogeneity analyses were conducted using the Q-test and I2 statistic.38
Publication bias and sensitivity analyses
Publication bias was assessed using funnel plots relating effect size to precision (inverse of standard error), two-tailed rank correlation tests,39 and trim and fill techniques,40 using the Comprehensive Meta-Analysis software, version 2.0. To provide protection against Type I error, publication bias was assessed only in those analyses which consisted of at least five studies. To evaluate the stability of results, we conducted sensitivity analyses by excluding samples consisting primarily of patients with known nonpsychiatric medical conditions (for example, CVD, migraine, pregnant samples), as the inflammatory processes could differ from those in the general population, despite study-level control. These post hoc models were fitted if there remained at least two studies for the analysis.
Study-level risk of bias
Risk of study-level bias was assessed with a checklist modeled after the Quality Assessment Tool for Quantitative Studies (QAT),41 modified slightly to fit our observational research question (see Supplementary Materials for details). We calculated both a categorical global rating (that is, high, moderate or low risk of bias), by using the QAT global rating instructions for relevant domains (selection bias, study design, control of relevant covariates, assessment validity, and use of appropriate data analysis techniques), and an average QAT score (average of the ordinal domain ratings). For each study, the percentage of recommended covariates (for example, age, sex, BMI, and the use of medications or other substances, among others)22 controlled either methodologically (that is, through exclusion or matching of subjects) or statistically was documented and entered as part of the QAT score (see Supplementary Material). Each domain was scored on a three-point likert scale (strong/moderate/weak); thus, both categorical and average QAT scores ranged between 1.0 and 3.0, with higher scores indicating greater potential for bias.
Meta-regression models
For each analysis, we tested the effect of average QAT scores using univariate models. We then conducted multivariate meta-regression models assessing the impact of a study's inclusion of symptomatic individuals, while statistically controlling for study-level bias. Finally, we ran a series of dummy-coded meta-regression models to assess the effect of psychiatric diagnosis or symptom type. Due to the small number of studies of non-PTSD psychiatric populations, we used models comparing samples of non-comorbid PTSD participants to all other samples (including those using other psychiatric diagnoses and nonpsychiatric samples). Any models that displayed significant differences between the two groups (PTSD vs other samples) were then subjected to post hoc analyses to determine which symptom subgroups differed significantly from the PTSD samples. To increase power and interpretability, all meta-regression models were fitted only if at least four samples were included in the analysis.
Results
Study selection
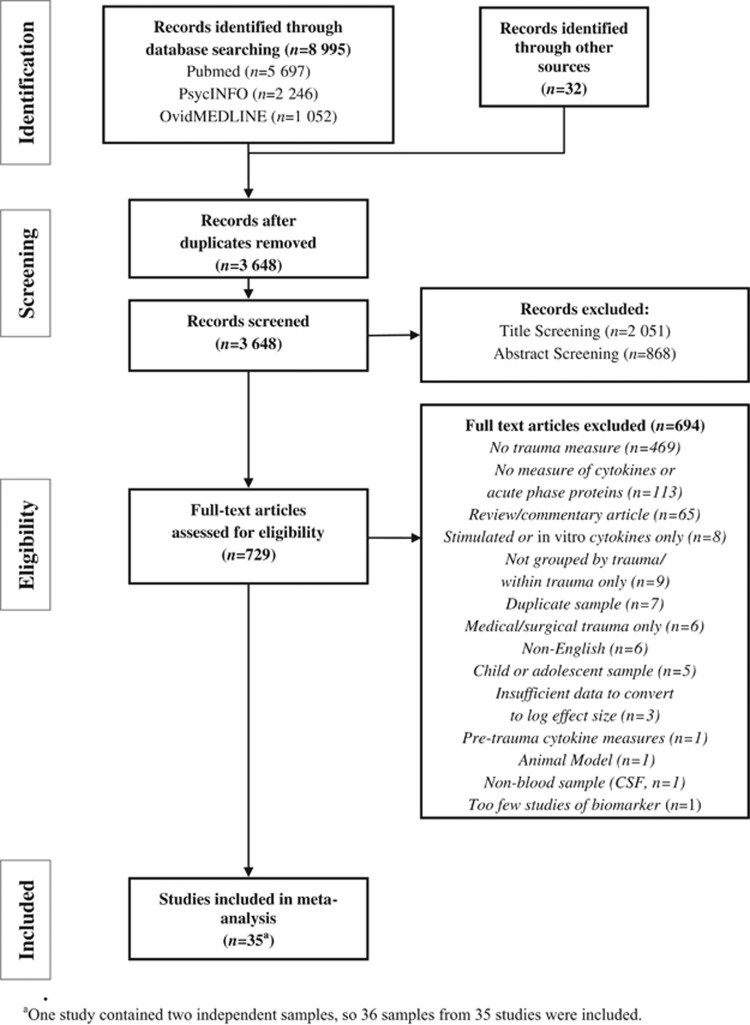
A total of 3647 unique articles were identified through our searches, the majority of which were excluded through title or abstract screening (n=2919). Reasons for exclusion of full-text articles are shown in Figure 1. We identified 40 independent samples measuring 29 different cytokines, receptors or acute-phase proteins that met initial inclusion criteria. Of these, only biomarkers measured by at least three samples were included in our analyses. Three studies that failed to provide sufficient information to calculate log-transformed effect size estimates were not included. Thus, nine biomarkers from 36 independent samples were included in the meta-analysis (see Table 1).
Figure 1.
Study screening and eligibility.
Table 1. Studies included in the meta-analysis.
| Study | Total N | Group n (T/NT) | Country | Type of study (Sample name) | Risk of biasa | %F | Age (years) M (s.d.) | Sample type | Biomarkers included | Biomarkers excluded |
|---|---|---|---|---|---|---|---|---|---|---|
| Spitzer, et al.42 | 3047 | 1653/1394 | Germany | Population study (SHIP): T/NT | Low | 52 | 54 (15) | Serum | CRP | |
| Baumert, et al.43 | 3012 | 1224/1788 | Germany | Population study (KORA): T/NT | Low | 52 | 56 (13) | Blood, N/S | CRP | IL-18 |
| Cho, et al.44 | 2716 | (Regress.) | USA | Population study (CARDIA): ELA | Low | 55 | Range 18–30 | Plasma | IL-6, CRP | |
| O'Donovan, et al.45 | 979 | (Regress.) | USA | Stable CVD: trauma | Low | 19 | 67 (11) | Serum | IL-6, TNF-α, CRP | Resistin |
| Danese, et al.46 | 886 | 315/551 | New Zealand | Prospective birth cohort (Dunedin): ELA/no ELA | Low | 47 | All 32 years | Blood, N/S | CRP, fibrinogen | |
| Slopen, et al. (C)28 | 822 | (Regress.) | USA | Population study, subsample (MIDUS)b: ELA | Low | 53 | 59 (12) | Serum | CRP, IL-6, fibrinogen | |
| Bertone-Johnson, et al.47 | 700 | 452/248 | USA | Population study (Nurses' health study): ELA/no ELA | Low | 100 | 44 (5) | Plasma | IL-6, CRP | TNFR2 |
| Rooks, et al.48 | 482 | 245/237 | USA | Population (VET twin registry)c: high/low ELA | Low | 0 | 55 (3) | Plasma | IL-6, CRP | |
| Plantinga, et al.49 | 476 | 108/368 | USA | Population (VET twin registry)c: PTSD/no PTSD | Low | 0 | 56 (3) | Plasma | Fibrinogen | IL-6, CRPd |
| Slopen, et al. (AA)28 | 177 | (Regress.) | USA | Population study (AA) (MIDUS+Mke.)e: ELA | Low | 67 | 54 (11) | Serum | CRP, IL-6, fibrinogen | |
| Tietjen, et al.50 | 141 | 90/51 | USA | Migraine patients (+HC): ELA/no ELA | Moderate | 100 | 33 (7) | Blood, N/S | CRP, IL-6, TNF-α | Adiponectin, TGF-β |
| Smith, et al.51 | 138 | 95/43 | USA | Primary care/GYN: 35 ELA+PTSD; 31 PTSD−ELA; 29 HC+ELA; 43 NTC | Moderate | 50 | 46 (11) | Plasma | IL-1β,IL-2, IL-4, IL-6, IL-10, TNF-α | IFN-α |
| Blackmore, et al.52 | 137 | 50/80 | USA | Pregnant women: T/NT | Low | 100 | 25 (4) | Serum | IL-6, TNF-α | |
| Gouin, et al.53 | 130 | 57/73 | USA | Dementia caregivers: ELA/no ELA | High | 82 | 65 (13) | Serum | IL-6, TNF-α, CRP | |
| Guo, et al.54 | 100 | 50/50 | China | PTSD/NTC | High | 53 | 42 (13) | Serum | IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α | |
| Carpenter, et al.55 | 92 | 21/71 | USA | ELA/no ELA | Moderate | 51 | 31 (9) | Plasma | CRP | |
| Gill, et al.56 | 77 | 53/24 | USA | 26 PTSD, current; 27 PTSD, recovery; 24 NTC | Low | 100 | 34 (8) | Plasma | IL-6, CRP | |
| Danielson, et al.57 | 75 | 40/35 | Canada | Undergraduate students: current IPV/no current IPV | Moderate | 100 | 20 (2) | Plasma | IL-6, IL-10 | |
| Cankaya, et al.58 | 75 | 64/11 | USA | Primary care: traumatic lossf/no loss | Low | 100 | ≥40 | Serum | IL-6 | IGF-1 |
| Dennison, et al.59 | 72 | 24/48 | Ireland | Schizophrenia: 24 PSY+ELA; 16 PSY−ELA; 32 NTC | High | 54 | 37 (10) | Plasma | IL-1β, IL-6, IL-8, TNF-α | |
| Tucker, et al.60 | 68 | 54/14 | USA | Hurricane or mixed trauma: T/NT | High | 35 | 36 (12) | Serum | IL-2, IL-6 | |
| Hepgul, et al.61 | 63 | 25/38 | UK | First episode psychosis: 10 PSY+ELA; 15 HC+ELA; 18 PSY−ELA; 20 HC−ELA | High | 29 | 27 (2) | Plasma | CRP | |
| Cohen, et al.62 | 61 | 48/13 | Israel | Prospective musculoskeletal injury: injured/NTC | Moderate | 28 | 35 (13) | Serum | IL-4, IL-6, IL-8, IL-10 | TGF-β |
| Gola, et al.63 | 60 | 22/25 | Germany | PTSD±MDD/HC | Low | 55 | 31 (10) | Plasma | IL-6, IL-8, IL-10, TNF-α | MCP-1 |
| Hoge, et al.64 | 56 | 28/28 | USA | PTSD/HC | Moderate | 50 | 41 (11) | Plasma | IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, TNF-α | IL-1α, IL-5, IL-7, IL-13, IL-15, IFN-γ, IP-10+others |
| Grassi-Oliveira, et al.32 | 49 | 30/19 (Regress.) | Brazil | MDD outpatients/HC: ELA regress. | Moderate | 100 | 38 (8) | Plasma | TNF-α | TNFR1, TNFR2 |
| Maes, et al.65 | 45 | 13/32 | Belgium | PTSD/HC | High | 80 | 46 (8) | Serum | IL-6 | sIL-6r, sIL-1RA |
| Janusek, et al.66 | 40 | (ELA regress.) | USA | Breast cancer | Low | 100 | 56 (9) | Plasma | IL-6 | |
| Symes, et al.67 | 39 | 25/14 | USA | CVD: IPV/no IPV | High | 100 | 57 (NR) | Serum | IL-6, CRP, TNF-α | |
| Spivak, et al.68 | 38 | 19/19 | Israel | PTSD/HC | Moderate | 0 | 29 (11) | Serum | IL-1β | sIL-2r |
| Gill, et al.69 | 36 | 22/14 | USA | PTSD±MDD/NTC | Moderate | 42 | 36 (12) | Plasma | IL-6 | |
| Groer, et al.70 | 31 | 15/16 | USA | Sexual assault crisis center: raped/HC | Moderate | 100 | Range 18–51 | Serum | IL-6 IL-10, CRP | IFN-γ |
| Pace, et al.71 | 28 | 14/14 | USA | MDD+ELA/NTC | Moderate | 0 | 30 (9) | Plasma | IL-6 | |
| Bonne, et al.72 | 26 | 15/11 | USA | PTSD/NTC | Moderate | 73 | 36 (12) | CSF | IL-6 | IGF-1 |
| Baker, et al.73 | 19 | 11/8 | USA | PTSD/HC | High | 0 | 42 (9) | Plasma | IL-6 |
Abbreviations: Blood, N/S, blood, not otherwise specified; ELA, early life adversity/childhood trauma; HC, healthy controls, not otherwise specified; IPV, intimate partner violence; MDD, major depressive disorder; N/S, not specified; NT, non-trauma exposed; NTC, non-trauma-exposed controls; primary care/GYN, recruited from primary care or gynecology clinics; PSY, psychosis; PTSD, posttraumatic stress disorder; regress., regression model (continuous outcome); T, trauma exposed; TC, trauma-exposed controls.
Risk of bias is based on the modified QAT.41 Overall risk of bias is based on selection bias, study design, percent of recommended covariates controlled,22 use of reliable and valid assessments and use of appropriate statistical analyses.
Caucasian subsample.
Based on analysis of individuals (not twin pairs) and had been described in original study as statistically corrected for clustering.
Subsample of Rooks et al.,49 so overlapping biomarkers were not included.
African American subsample: comprised of MIDUS and Milwaukee (Mke.) samples.
Sudden loss, as defined by TLEQ.74
None of the meta-analysis models for the anti-inflammatory cytokines (IL-4 and IL-10) was statistically significant (see Supplementary Figure 1), so we focus on presenting analyses of the acute-phase proteins (CRP and fibrinogen) and the proinflammatory cytokines (IL-1β, IL-2, IL-6, IL-8, and TNF-α).
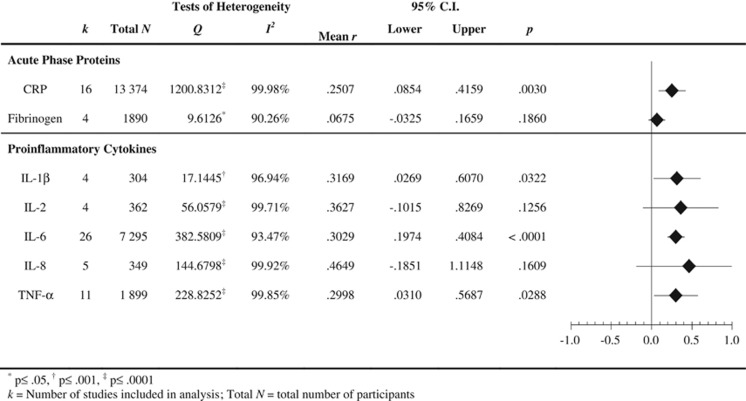
The results of the baseline models, based on 36 independent samples, are presented in Figure 2. As expected, there was evidence of significant heterogeneity across studies for all biomarkers. Although rank correlation tests revealed a significant correlation between the standardized effect size and the standard error for IL-6 (τ=0.2963, z=2.1889, P=0.0302), no imputed ‘missing studies' were identified using the trim and fill method.40
Figure 2.
Meta-analysis summary statistics.
Acute phase proteins
CRP
Analyses for CRP included 16 studies and a total of 13 374 participants. As shown in Figure 2, a significant association between CRP concentration and trauma exposure was detected (mean r=0.2507, P=0.0030).
Fibrinogen
Analysis of four studies involving 1890 participants showed no significant correlation between trauma exposure and fibrinogen (mean r=0.0675, P=0.1860; see Figure 2).
Proinflammatory cytokines
IL-1β
Meta-analysis of four studies (304 participants) showed a significant relationship between IL-1β and trauma exposure (mean r=0.3169, P=0.0322), displayed in Figure 2.
IL-2
Overall analyses of four studies (362 participants) revealed no statistically significant association between IL-2 and trauma exposure (mean r=0.3627, P=0.1256; see Figure 2).
IL-6
As shown in Figure 2 analysis of 26 studies with 7295 participants showed a significant relationship between trauma exposure and IL-6 (mean r=0.3029, P<0.0001).
IL-8
No significant overall correlation relating trauma exposure to IL-8 was detected, based on five studies with 349 participants (mean r=0.4649, P=0.1609; see Figure 2).
TNF-α
As presented in Figure 2 TNF-α was significantly associated with trauma exposure in analysis of 11 studies with 1899 participants (mean r=0.2998, P=0.0288).
Meta-regression models and sensitivity analyses
As shown in Table 2, risk of study-level bias (defined as the average of the applicable QAT domains: selection bias, study design, covariate control, assessment reliability/validity, and statistical analysis) was a significant predictor of heterogeneity in meta-regression models for the proinflammatory cytokines IL-6 and IL-8, such that higher risk of bias was associated with larger effect size estimates. In models controlling for risk of bias, studies that included participants with psychiatric disorders yielded larger effects for IL-1β and IL-6.
Table 2. Meta-regression models.
| k | Total N |
QATa |
QAT+symptoms |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | df | R2 | Covariates | β | Q | df | R2 | Covariates | β | |||
| Acute phase proteins | ||||||||||||
| CRP | 16 | 13 374 | 0.6865 | 1.14 | 0.0436 | QAT | −0.2088 | 1.6658 | 2.13 | 0.1015 | QAT Symptoms | −0.2832 −0.2513 |
| Fibrinogen | 4 | 1890 | 0.0010 | 1.2 | 0.0008 | QAT | −0.0284 | —b | ||||
| Proinflammatory cytokines | ||||||||||||
| IL-1β | 4 | 304 | 0.1287 | 1.2 | 0.0598 | QAT | −0.2446 | 16.6775† | 2.1 | 0.9728 | QAT Symptoms | −0.2522 1.0175‡ |
| IL-2 | 4 | 362 | 1.5621 | 1.2 | 0.4690 | QAT | 0.6848 | 0.8805 | 2.1 | 0.4682 | QAT Symptoms | 0.6657 0.0257 |
| IL-6 | 26 | 7295 | 17.0306‡ | 1.25 | 0.2773 | QAT | 0.5266‡ | 31.6302‡ | 2.24 | 0.3845 | QAT Symptoms | 0.3812** 0.3568** |
| IL-8 | 5 | 349 | 4.9369* | 1.3 | 0.6327 | QAT | 0.7954* | 3.6573 | 2.2 | 0.6500 | QAT Symptoms | 0.7479 0.1426 |
| TNF-α | 11 | 1899 | 3.6164 | 1.9 | 0.2454 | QAT | 0.4954 | 3.5195 | 2.8 | 0.2512 | QAT Symptoms | 0.4455 0.0937 |
Abbreviations: k, number of studies included in analysis; Q, test statistic (F-analog) of the variability explained by the meta-regression model; R2, coefficient of determination for meta-regression model (variance explained by model); Total N, total number of participants; β, standardized regression coefficient.
*P⩽0.05, **P⩽0.01, †P⩽0.001, ‡P⩽0.0001.
QAT=Quality Assessment Tool:41 these analyses used an average score including selection bias, study design, percent of relevant covariates controlled, use of reliable and valid assessments, and use of appropriate data analysis techniques.
The studies in this analysis did not include any symptomatic groups of participants.
None of the meta-regression models comparing PTSD samples with all other samples was statistically significant, with the exception of fibrinogen (which did not include any psychiatric samples) and IL-6 (see Supplementary Table 2). Post hoc comparisons coding non-PTSD psychiatric samples separate from asymptomatic samples revealed no differences in effect sizes for IL-6 between non-comorbid PTSD vs other types of psychiatric disturbance.
Sensitivity analyses showed that the findings were robust to exclusion of medical samples, which did not significantly change the pattern or significance of findings for any of the biomarkers except TNF-α (see Supplementary Figure 1). Although of increased magnitude, the TNF-α mean effect size was no longer statistically significant, once medical samples were excluded (k=6, mean r=0.4579, P=0.0857).
Discussion
To our knowledge, this is the first meta-analysis of trauma exposure as a risk factor for inflammation to utilize a transdiagnostic perspective. We integrated all available studies measuring trauma exposure, rather than limiting the analysis to a single diagnostic category. Further, to reflect the true status of the current literature, we incorporated both continuous and categorical measures. Although this procedure is likely to increase the observed heterogeneity in effect sizes across studies, it is essential to represent the diversity of study designs to obtain an accurate and comprehensive review of inflammation in trauma survivors. A growing body of evidence suggests that there may be a dose-response relationship between physical health problems and increased levels of exposure to traumatic events.75,76 Within the field of trauma research, however, it has been difficult to arrive at a single adequate method of quantifying cumulative trauma exposure, taking into account differences in number, frequency and severity of potentially traumatic experiences. As a result, there was insufficient literature to assess the potential impact of continuous vs categorical measures or to assess variability across different continuous measures of trauma exposure.
As expected, increased inflammation in trauma-exposed samples was found across a range of biomarkers. In particular, we noted moderate-to-large correlations relating trauma exposure to circulating concentrations of the proinflammatory cytokines IL-1β, IL-6, and TNF-α and of the acute-phase protein CRP. On the basis of our meta-regression models, the relationship between trauma exposure and the proinflammatory cytokines IL-1β and IL-6 was especially pronounced in samples that included, at least in part, clinical populations, as compared with samples which did not specifically recruit symptomatic individuals. No significant differences were observed as a function of the specific psychiatric diagnosis, although the statistical power was likely insufficient, at least in most analyses, to detect such relationships if they exist.
Because it was not possible to obtain log-transformed effect sizes for all studies, we produced log-transformed effect size estimates following accepted techniques.31 When we were unable to confirm with authors the base of the logarithm applied, we assumed the use of log-10 transformations, in accordance with similar studies. In keeping with prior recommendations for biomarker research,77 we advocate for greater consistency in reporting of methods, including transformations applied, sample demographics and characteristics, and covariates controlled in statistical analyses. Although it is beyond the scope of this study to recommend specific statistical techniques, other authors have endorsed the use of log-transformation when data are log-normally distributed, due to its flexibility in allowing for the use of traditional (parametric) statistical techniques and due to the ability to back-transform (exponentiate) geometric means to facilitate interpretation and comparison.77
Despite recent interest in these questions, research on the relationships among trauma exposure, psychiatric symptomatology, and inflammatory biomarkers has historically been limited to the PTSD literature. Thus, our power to detect differences between diagnostic groups was somewhat limited by a relative scarcity of reporting or measurement of trauma histories in non-PTSD psychiatric samples. Given the evidence that trauma exposure, particularly when prolonged and/or occurring early in life, is associated with a wide range of chronic medical and psychological health problems2,26 even after accounting for the effects of psychiatric disorders,75,76 it would be useful for future research to routinely include measures of trauma exposure in both medical and psychiatric populations.
Our analyses were also limited by the number of published biomarker studies providing information on participants' trauma history. Although the numbers of studies included in our meta-analyses are comparable with other early meta-analyses on inflammation and psychiatric disorders,14, 15, 16, 18, 19,20, 21 further research is necessary to determine whether replicable relationships exist between trauma exposure and those biomarkers with relatively few published studies (for example, fibrinogen, IL-1β, IL-2, and IL-8).
Our findings suggest that the risk of study-level bias, especially related to the control of relevant covariates,22 such as medication use, BMI, and comorbid medical conditions, was significantly related to heterogeneity of effect sizes observed across studies. That is, higher QAT scores (indicating greater risk of bias) were associated with larger correlations between trauma exposure and IL-4, IL-6, IL-8 and IL-10. We therefore advocate for wider adoption of accepted standards for control of these potentially relevant covariates in future studies.
Another important question concerns the longitudinal course of inflammation in trauma-exposed individuals. We were unable to evaluate the effects of treatment or to examine the longitudinal course of inflammation following traumatic events in our analyses. However, there is preliminary evidence56,72,78,79 to suggest that successful treatment of trauma-related psychopathology may lead to improvements in the chronic immune dysregulation observed in our analyses, although results to date have been somewhat contradictory and unclear. This lack of consistency may be due, at least in part, to methodological differences among studies. Further research is needed to better understand the time course of inflammatory biomarkers following successful psychological or pharmacological treatment.
Conclusions
The relevance of inflammation in the pathophysiology and consequences of psychiatric disorders and general medical conditions has been increasingly recognized within research, clinical and public health arenas.9,10,80,81 The results of this meta-analysis are in keeping with a growing body of cross-disciplinary evidence11,12,82 which provides a framework for examination of transdiagnostic relationships among psychiatric risk factors (such as trauma exposure) and both psychological and physiological dysfunction. In our review of 36 samples with 14 991 participants, we found moderate correlations between inflammatory biomarker concentrations (IL-1β, IL-6, TNF-α, and CRP) and trauma exposure (mean rs=0.2455, 0.3067, 0.2890, and 0.2998, respectively) across 36 independent samples with a total of 14 991 participants. Further research is needed to confirm this association in a broader range of psychiatric and general medical populations, and to determine whether these findings extend to other inflammation-related biomarkers.
Although prior systematic reviews on inflammatory biomarkers in PTSD have provided a qualitative synthesis of the literature,4,5,81,83, 84, 85 to our knowledge, this is the first meta-analysis to examine the relationship of trauma to proinflammatory cytokines and acute-phase proteins. Meta-analyses of inflammatory activity observed within other psychiatric disorders (for example, depression14,18, 19, 20, 21 and bipolar disorder15,16) have found evidence of systemic inflammation, but none have yet examined the impact of trauma on these relationships. Our findings are also consistent with the results of two recent studies59,61 (included within our analysis), which found significantly higher inflammatory biomarkers only in those psychotic patients with a trauma history, as compared with healthy control participants. In both studies, patients without a trauma history did not significantly differ from controls. However, the majority of studies in psychiatric populations that we screened did not assess participants' histories of trauma exposure, so further research is needed to confirm and clarify these findings. We therefore endorse routine assessment and reporting of trauma exposure within immunological research studies.
Acknowledgments
This research was supported by the Canadian Institute of Military and Veteran Health Research and by a Chancellor's Faculty Research and Development Grant from Nova Southeastern University. We thank all the primary authors who gave information regarding their articles, including the following individuals who provided data for this project: E Bertone-Johnson, M Cohen, LW Janusek, JK Kiecolt-Glaser, V Mondelli and N Hepgul, TWW Pace and CM Heim, KJ Ressler and AK Smith, N Slopen, C Spitzer, GE Tietjen and J Khubchandani, R von Känel, and A Woods.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett KA. Psychological trauma and physical health: a psychoneuroimmunology approach to etiology of negative health effects and possible interventions. Psychol Trauma. 2009;1:35–48. [Google Scholar]
- Schauer M, Elbert T. Dissociation following traumatic stress. Z Psychol/J Psychol. 2010;218:109–127. [Google Scholar]
- Gill JM, Szanton S. Inflammation and traumatic stress: the society to cells resiliency model to support integrative interventions. J Am Psychiatr Nurses Assoc. 2011;17:404–416. doi: 10.1177/1078390311418652. [DOI] [PubMed] [Google Scholar]
- Baker DG, Nievergelt CM, O'Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Schiavone S, Jaquet V, Trabace L, Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal. 2013;18:1475–1490. doi: 10.1089/ars.2012.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stertz L, Magalhães PVS, Kapczinski F. Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry. 2013;26:19–26. doi: 10.1097/YCO.0b013e32835aa4b4. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target. Expert Rev Neurother. 2012;12:1143–1161. doi: 10.1586/ern.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2013;35:578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Miller GA, Rockstroh B. Endophenotypes in psychopathology research: where do we stand. Annu Rev Clin Psychol. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health (2011) NIMH Research Domain Criteria (RDoC)Retrieved Nov 30, 2013 from www.nimh.nih.gov/research-priorities/rdoc/nimh-research-domain-criteria-rdoc.shtml .
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun. 2012;26:1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2013;144:16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Tursich M, Ma M, Malcolm L, Greenbarg R. Metabolic, autonomic and immune markers for cardiovascular disease in posttraumatic stress disorder. World J Cardiol. 2014;6:455–461. doi: 10.4330/wjc.v6.i6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC-M, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2 R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Bizik G. Meta-analysis of plasma interleukine-6 levels in patients with depressive disorder. Activitas Nervosa Superior. 2010;52 (2:76–80. [Google Scholar]
- O'Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilic D, Cant AJ, Abinun M, Calvert JE, Spickett GP. Cytokine production differs in children and adults. Pediatr Res. 1997;42:237–240. doi: 10.1203/00006450-199708000-00018. [DOI] [PubMed] [Google Scholar]
- Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013;2013:434010. doi: 10.1155/2013/434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF. The health and social impact of growing up with adverse childhood experiences: The human and economic costs of the status quo. Centers for Disease Control and Prevention. 2007.
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders5th ednAmerican Psychiatric Association: Arlington, VA, USA; 2013 [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, et al. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med. 2010;72:694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HM, Hedges LV, Valentine JC.The Handbook of Research Synthesis and Meta-analysis2nd ednRussell Sage Foundation: New York, NY, USA; 2009 [Google Scholar]
- Yeh ETH, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in Cardiology. Circulation. 2003;107:370–371. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
- Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–6092. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Brietzke E, Pezzi JC, Lopes RP, Teixeira AL, Bauer ME. Increased soluble tumor necrosis factor-α receptors in patients with major depressive disorder. Psychiatry Clin Neurosci. 2009;63:202–208. doi: 10.1111/j.1440-1819.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol. 2005;90:175–181. doi: 10.1037/0021-9010.90.1.175. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. SAGE Publications: Thousand Oaks, CA, USA; 2001. [Google Scholar]
- Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. 2011.
- Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric "trim and fill" method of accounting for publication bias in meta-analysis. J Am Stat Asso. 2000;95:89–98. [Google Scholar]
- Effective Public Health Practice Project (2009). Quality Assessment Tool for Quantitative Studies.
- Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Baumert J, Lukaschek K, Kruse J, Emeny RT, Koenig W, von Kanel R, et al. No evidence for an association of posttraumatic stress disorder with circulating levels of CRP and IL-18 in a population-based study. Cytokine. 2013;63:201–208. doi: 10.1016/j.cyto.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Bower JE, Kiefe CI, Seeman TE, Irwin MR. Early life stress and inflammatory mechanisms of fatigue in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun. 2012;26:859–865. doi: 10.1016/j.bbi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin M-T, O'Farrelly C, et al. Clinical anxiety, cortisol and interleukin-6: Evidence for specificity in emotion–biology relationships. Brain Behav Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med. 2012;43:611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks C, Veledar E, Goldberg J, Bremner JD, Vaccarino V. Early trauma and inflammation: role of familial factors in a study of twins. Psychosom Med. 2012;74:146–152. doi: 10.1097/PSY.0b013e318240a7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, et al. Association between posttraumatic stress disorder and inflammation: A twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietjen GE, Khubchandani J, Herial NA, Shah K. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache. 2012;52:920–929. doi: 10.1111/j.1526-4610.2012.02165.x. [DOI] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B. 2011;156B:700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O'Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic Medicine. 2011;73:656–663. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med. 2012;44:287–292. doi: 10.1007/s12160-012-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu T, Guo J-C, Jiang X-L, Chen F, Gao Y-S. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med. 2012;5:323–325. doi: 10.1016/S1995-7645(12)60048-0. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Price LH. C-reactive protein, early life stress, and wellbeing in healthy adults. Acta Psychiatr Scand. 2012;126:402–410. doi: 10.1111/j.1600-0447.2012.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Lee H, Rotolo S, Szanton S. Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. J Psychosom Res. 2013;74:301–306. doi: 10.1016/j.jpsychores.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Danielson AM, Matheson K, Anisman H. Cytokine levels at a single time point following a reminder stimulus among women in abusive dating relationships: Relationship to emotional states. Psychoneuroendocrinology. 2011;36:40–50. doi: 10.1016/j.psyneuen.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Cankaya B, Chapman BP, Talbot NL, Moynihan J, Duberstein PR. History of sudden unexpected loss is associated with elevated interleukin-6 and decreased insulin-like growth factor-1 in women in an urban primary care setting. Psychosom Med. 2009;71:914–919. doi: 10.1097/PSY.0b013e3181be7aa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison U, McKernan D, Cryan J, Dinan T. Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychol Med. 2012;42:1865–1871. doi: 10.1017/S0033291712000074. [DOI] [PubMed] [Google Scholar]
- Tucker P, Jeon-Slaughter H, Pfefferbaum B, Khan Q, Davis NJ. Emotional and biological stress measures in Katrina survivors relocated to Oklahoma. Am J Disaster Med. 2010;5:113–125. doi: 10.5055/ajdm.2010.0013. [DOI] [PubMed] [Google Scholar]
- Hepgul N, Pariante CM, Dipasquale S, DiForti M, Taylor H, Marques TR, et al. Childhood maltreatment is associated with increased body mass index and increased C-reactive protein levels in first-episode psychosis patients. Psychol Med. 2012;42:1893–1901. doi: 10.1017/S0033291711002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med. 2011;42:117–131. doi: 10.2190/PM.42.2.b. [DOI] [PubMed] [Google Scholar]
- Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin A-h, Delmeire L, Gastel AV, Kenis G, de Jongh R, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Janusek LW, Tell D, Albuquerque K, Mathews HL. Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain Behav Immun. 2013;30:S149–S162. doi: 10.1016/j.bbi.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes L, McFarlane J, Frazier L, Henderson-Everhardus MC, McGlory G, Watson KB, et al. Exploring violence against women and adverse health outcomes in middle age to promote women's health. Crit Care Nurs Q. 2010;33:233–243. doi: 10.1097/CNQ.0b013e3181e6d7c4. [DOI] [PubMed] [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, et al. Elevated levels of serum interleukin-1β in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- Gill JM, Luckenbaugh D, Charney D, Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol Psychiatry. 2010;68:999–1006. doi: 10.1016/j.biopsych.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Groer MW, Thomas SP, Evans GW, Helton S, Weldon A. Inflammatory effects and immune system correlates of rape. Violence Vict. 2006;21:796–808. [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Bonne O, Gill JM, Luckenbaugh DA, Collins C, Owens MJ, Alesci S, et al. Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance P in the cerebrospinal fluid of civilians with posttraumatic stress disorder before and after treatment with paroxetine. J Clin Psychiatry. 2011;72:1124–1128. doi: 10.4088/JCP.09m05106blu. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, et al. Plasma and cerebrospinal fluid Interleukin-6 concentrations in Posttraumatic Stress Disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kasplan AS, Watson SB, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the traumatic life events questionnaire. Psychol Assess. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Scott KM, Koenen KC, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Benjet C, et al. Associations between lifetime traumatic events and subsequent chronic physical conditions: a cross-national, cross-sectional study. PLoS One. 2013;8:e80573. doi: 10.1371/journal.pone.0080573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husarewycz MN, El-Gabalawy R, Logsetty S, Sareen J. The association between number and type of traumatic life experiences and physical conditions in a nationally representative sample. Gen Hosp Psychiatry. 2014;36:26–32. doi: 10.1016/j.genhosppsych.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Olivier J, Johnson WD, Marshall GD. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Annals of allergy. Asthma Immunol. 2008;100:333–337. doi: 10.1016/S1081-1206(10)60595-9. [DOI] [PubMed] [Google Scholar]
- Tursich M. Relationships Between Psychological Distress and Immune Function in Women with a History of Childhood Maltreatment. PhD thesis, Nova Southeastern University: Fort Lauderdale, FL, USA; 2012. [Google Scholar]
- Wilson DR, Vidal B, Wilson WA, Salyer SL. Overcoming sequelae of childhood sexual abuse with stress management. J Psychiatr Ment Health Nurs. 2012;19:587–593. doi: 10.1111/j.1365-2850.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain Behav Immun. 2012;26:239–250. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Andrews JA, Neises KD. Cells biomarkers, and post-traumatic stress disorder: evidence for peripheral involvement in a central disease. J Neurochem. 2012;120:26–36. doi: 10.1111/j.1471-4159.2011.07545.x. [DOI] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R. Interplay between neuroimmunoendocrine systems during post-traumatic stress disorder: a minireview. Neuroimmunomodulation. 2010;17:192–195. doi: 10.1159/000258721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.