Figure 8.
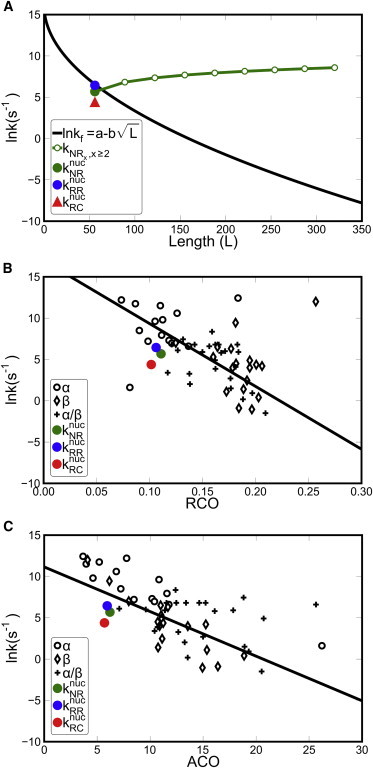
Comparison of CARP folding rates with values predicted based on chain length and topology. (A) CARP folding rates increase with chain length, in contrast to the decrease observed for globular proteins (31,32). The black solid line shows the negative square-root dependence obtained by fitting to experimentally determined folding rates (lnkf = 16.15–1.28L1/2 (32)). The green line shows the overall folding rate constants expected for NRx (x ≥ 2) by addition of nucleation rate constants for each available path for folding (i.e., kf(NRx) = knucNR + (x−1) knucRR). Solid circles show nucleation rates for NR (green), RR (blue), and RC (red). (B and C) Comparison of folding rates estimated by relative contact order (RCO, B) and absolute contact order (ACO, C) with CARP nucleation rates. Solid symbols show experimentally determined folding rate constants (lnkf) for globular proteins with different secondary structure composition (○, all α; ⋄, all β; +, α/β). Lines show linear fits between RCO (B) and ACO (C) and lnkf for all globular proteins, regardless of secondary structure. To see this figure in color, go online.
