Figure 1.
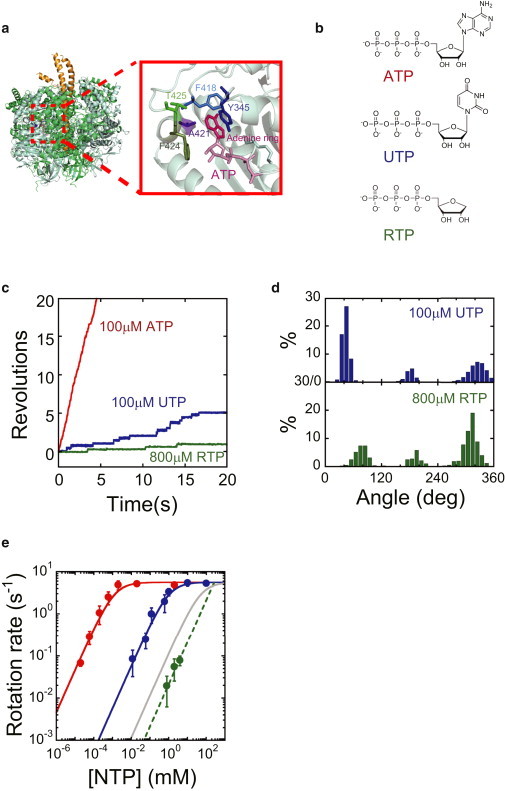
Rotary motion of F1 in the presence of UTP or RTP. (a) Side view of the crystal structure of F1 (PDB code 1BMF): interface between αDP and βDP. The α, β, and γ subunits are shown in green, light green, and yellow, respectively. (b) Structural formulas for ATP, UTP, and RTP. (c) Time courses of rotary motion of wild-type F1 in the presence of 100 μM ATP (red), 100 μM UTP (blue), and 800 μM RTP (green). (d) Histogram of the angular position during rotation, calculated from Fig. 1c. (e) Rotational velocity (V) at various NTP concentrations. Red, blue, and green points represent the rotational velocity in the presence of ATP, UTP, and RTP, respectively. The solid curves represent Michaelis-Menten fits with V = Vmax[ATP]/([ATP]+Km), where VmaxATP = 5.6 s−1, VmaxUTP = 5.6 s−1, KmATP = 1.2 μM, and KmUTP = 9.1 × 102μM. From these fits, the rate constants for ATP and UTP binding were calculated as kon = 3 × Vmax/Km, with konATP = 1.4 × 107 M−1 s−1, and konUTP = 1.8 × 104 M−1 s−1. The gray curve represents the simulated Michaelis-Menten curve produced by possible ATP contamination. The dashed curve represents the linear fit with V = 1/3 × konRTP × [RTP], where konRTP = 6.3 × 101 M−1 s−1. To see this figure in color, go online.
