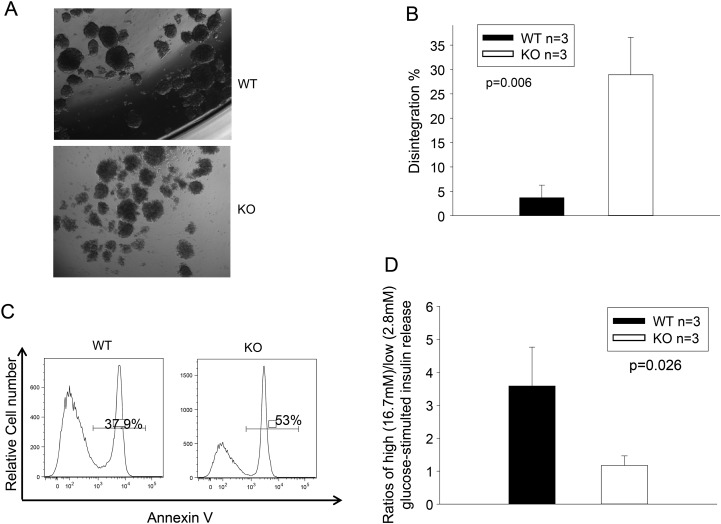
Figure 2.
TGFBI KO islet survival and function is compromised in vitro. (A and B) TGFBI KO islets quickly lose their integrity in culture. Freshly isolated WT and TGFBI KO islets were cultured in F-12K serum-free medium containing 2% BSA and 1X MEM non-essential amino acids for 24 h. Their morphology is illustrated in micrographs (A). About 150–200 islets from each mouse were assessed visually, and the percentages of those that lost integrity (defined as having lost 50% of capsule surrounding the islets) were registered. Means ± SD of the percentage of each group (based on three independent experiments) are illustrated (B), and statistical significance of the difference is indicated (P = 0.006; Student's t test). (C) TGFBI KO islets are prone to apoptosis in culture. WT and KO islets were cultured in serum-free medium for 24 h, as described above, dispersed and stained with annexin V, followed by flow cytometry analysis. Percentages of annexin V-positive cells are indicated. The experiment was performed 3 times, and histograms of a representative experiment are shown, in which the WT and KO islet cells showed 37.9% and 53% apoptosis, respectively. (D) TGFBI KO islet function is compromised after culture. WT and KO islets were cultured in serum-free medium for 24 h. Ten islets were picked and stimulated sequentially with low- and high-glucose concentrations (2.8 and 16.7 mm, respectively). Insulin released into medium after stimulation was assessed by ELISA in duplicate samples. Ratios of released insulin upon low- and high-glucose stimulation were calculated. Means ± SD of the ratios of each group (from three independent experiments) are reported, and statistically significant differences are indicated (P = 0.026; Student's t test).