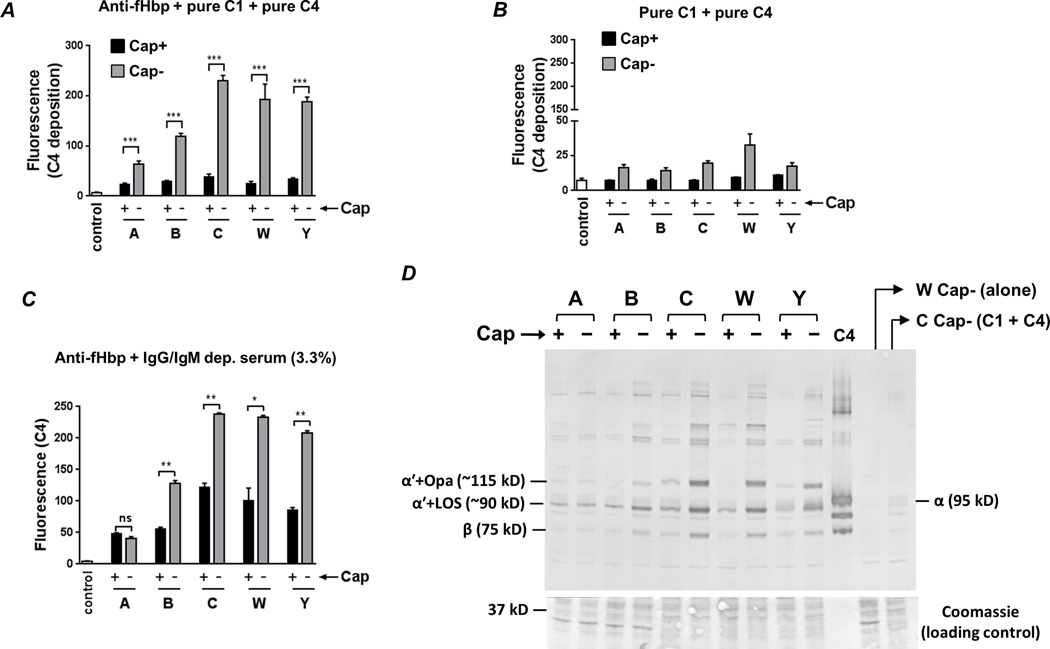
Fig. 2.
Capsule expression limits anti-fHbp−mediated C4b deposition. A. Bacteria were incubated with anti-fHbp (10 µg/ml), purified C1 complex (7.5 µg/ml) and pure C4 (50 µg/ml) and C4b deposited on bacteria (median fluorescence) was measured by flow cytometry. ‘Control’ represents the fluorescence of a reaction mixture that lacked complement components. The median fluorescence of each reaction was recorded and each bar represents the mean (SEM) of 3 experiments (Supplemental Figure S1 shows representative histograms). Y-axis, median fluorescence of C4 deposition. ***, P<0.001 (ANOVA). B. C4b deposition on meningococci is Ab-dependent. Bacteria were incubated with C1 and C4 in the absence of anti-fHbp and C4b deposition was measured by flow cytometry. Axes and controls are as described in A (note the magnified lower half of the Y-axis compared to panel A). C. Capsule inhibits anti-fHbp−mediated C4b deposition in the presence of human serum. Isogenic capsule mutants were incubated with anti-fHbp, followed by the addition of IgG and IgM depleted human serum at a final concentration 3.3%. Axes are as described above. **, P<0.01; *, P<0.05; ns, not significant (ANOVA). D. Western blotting confirms capsule-mediated inhibition of C4b deposition and identifies LOS and opacity proteins (Opa) as targets for C4b deposited by anti-fHbp. Bacteria were incubated with anti-fHbp, C1 and C4 as described in A. The ~87 kD α' chain of C4b binds to its bacterial targets covalently though ester or amide bonds. Electrophoresis under reducing conditions results in the ~87 kD α' migrating as a complex with its bacterial targets. Proteins were transferred to a PVDF membrane by western blotting to reveal C4b α'-bacterial target complexes. The locations of the ~90 kD and ~115 kD complexes of (LOS + C4b α'; labeled “α'+LOS (~90 kD)”) and (Opa + C4b α'; labeled “α'+Opa (~115 kD)”), respectively, are indicated; the identity of these bands was defined previously (41). The lane marked ‘C4’ contains pure C4 and the positions of the intact 95 kD α (labeled “α (95 kD)”) and 75 kD β (labeled “β (75 kD)”) chains are shown. Control lanes contained bacteria alone (labeled ‘W Cap− (alone)), or bacteria plus C1 and C4 with no added anti-fHbp. Proteins migrating below the 50 kD marker were stained with coomassie blue to ensure similar protein loading across the isogenic Cap+ and Cap− mutants.