Abstract
Background
Exposures of children to phthalates, parabens, and bisphenol-A (BPA) are of concern because of their hormonal potential. These agents are found in a wide range of foods and packaging. We investigated whether intake of certain foods predict exposures to these chemicals in young girls.
Methods
Among 1101 girls (6–8 years at enrollment) from the Breast Cancer and Environment Research Program (BCERP) study, we measured urinary exposure biomarkers for phthalates, parabens, and BPA and assessed dietary intake using 24-hour recall 2–4 times. We examined the average daily servings of major and minor food groups categorized as 0- <0.5, 0.5 – < 1 and ≥ 1 servings per day. Items included dairy, eggs, fats, fish, fruit, single grains, meat, non-poultry meats, pasta, poultry and vegetables. Covariate-adjusted least squares geometric means and 95% confidence intervals of creatinine-corrected phthalate and phenol metabolite concentrations in urine were calculated in relation to food intake.
Results
Grains, flour and dry mixes and total fish consumption were positively associated with BPA and the sum of four di-2-ethylhexylphthalate (DEHP) urinary metabolite concentrations. Non-fresh vegetables and poultry were both positively associated with BPA and paraben urinary concentrations. Fats, oils and poultry consumption were positively associated with BPA. Whole-fat dairy consumption was associated with ΣDEHP.
Conclusions
Some foods may contribute to child exposures to certain chemicals, and this may constitute modifiable means to reduce these environmental exposures.
Keywords: biomarkers, endocrine disruptors, phthalates, bisphenolA, parabens
1. Introduction
Phthalates, bisphenol A (BPA), and parabens are chemicals widespread in our environment, derived from many sources. Human exposure can occur through inhalation, ingestion, and dermal contact. Oral and inhalation pathways lead to much more efficient uptake of phthalates into the human body than the dermal pathways (Wormuth et al. 2006), making diet an important route of exposure (Lakind and Naiman 2011). Phthalates in plastic food packaging and canned food linings are not chemically bound to the products so they can be leached into foods (Cao et al. 2010; Kang and Kondo 2002). Measurable levels of both phthalates and BPA have been identified in a wide range of foods including baby food, canned beans and meat (Fromme et al. 2007; Schecter et al. 2013; Tsumura et al. 2001) and increased levels of their metabolites in humans have been associated with certain kinds of foods and packaging including poultry, canned vegetables, poultry, dairy and soda (Colacino et al. 2010; Schettler 2006) (Braun et al. 2011; Lakind and Naiman 2011; Trasande et al. 2013)
Phthalates are additives in many common consumer products such as food packaging, vinyl flooring, and personal care products (e.g., fragrances, cosmetics). BPA is used in the production of epoxy resins and polycarbonate plastics. BPA is found in canned and plastic food and drink packaging, dental sealants, adhesives and varnishes. Parabens have been used as preservatives in foods, drugs and cosmetics for over 50 years. They have effective antimicrobial activity and relatively low toxicity in humans (Darbre and Harvey 2008; Soni et al. 2001; Soni et al. 2002). Methyl paraben (MP) and propyl paraben (PP) are the two most common commercially-used parabens (Andersen et al. 2010; Soni et al. 2001; Soni et al. 2002).
Phthalates, BPA, and parabens are all considered endocrine disruptors (EDs); such compounds can mimic endogenous hormones, antagonize hormone function, alter the synthesis and metabolism of natural hormone or modify hormone receptors (Diamanti-Kandarakis et al. 2009). Exposure to EDs has been shown to adversely affect a range of human health endpoints, including reproductive function and thyroid activity in both males and females (Barlow and Foster 2003; Hauser et al. 2006; Latini et al. 2003; Meeker et al. 2011).
Measurable concentrations of phthalate metabolites, BPA and parabens are detected in the urine of over 90% of the U.S. population (CDC 2009). Most phthalates and BPA concentrations are normally higher in children and minority groups (Calafat et al. 2010), while DEP and paraben concentrations are generally higher in adults. We examined whether urinary biomarker levels were associated with increased intake of certain types of foods in a population of young girls. Furthermore, previous research has shown increased chemical exposure in humans associated with food packaging (Mariscal-Arcas et al. 2009; Vandenberg et al. 2007). Therefore, we also examined whether chemical exposure differed by specific types of food packaging and preparation. Identification of food sources will provide a way to reduce exposure to these chemicals through avoidance of these foods.
2. Materials and Methods
The Breast Cancer and Environment Research Program (BCERP) study population of girls 6–8 years at enrollment (2004–2007) in three U.S. sites, New York City, Cincinnati, and San Francisco Bay Area, has been previously described (Biro et al. 2010). Briefly, Mount Sinai School of Medicine (NYC), recruited through clinics, schools, and neighborhood centers in East Harlem, New York; Cincinnati Children’s Hospital/University of Cincinnati (Cincinnati), recruited through schools in the Cincinnati metropolitan area and through the Breast Cancer Registry of Greater Cincinnati; and the San Francisco Bay Area group recruited Kaiser Permanente Northern California Health Plan members in the San Francisco Bay Area. For this analysis, 1101 girls with baseline urinary biomarker measurements, diet, anthropometric and questionnaire data were included.
2.1 Dietary Data
Twenty-four hour dietary recalls for all participants were performed at the Cincinnati Center for Nutritional Research and Analysis using the Nutrition Data System for Research (NDSR). Regarding usual intake, there is evidence that the detailed information obtained from 24 hour diet recalls may provide more accurate estimated than information from FFQ’s (Schatzkin et al. 2003). Dietary recalls were conducted with participant caregivers over the telephone every 3 months during the first year after enrollment. Girls with two - four 24-hour dietary recalls collected during the year after baseline interview were included; values were averaged to estimate daily (24 hour) intake in grams. This was converted to servings which is the unit of analysis hereafter. Dietary recalls where total kilocalories were <400 or >4000 were considered outliers and excluded (n=18) (Willett 1998). Similarly, diet recalls that were completed more than a year after the baseline urine sample were excluded (n=23). Remaining girls after these exclusions were included in this analysis (n=1101).
Food groups were based on the USDA MyPyramid equivalents (United States Department of Agriculture 2010) and NDSR food groupings (Table 1). We calculated the average daily servings of the following food groups: dairy (including low and whole fat), eggs, fats (including vegetable oils, olive oil, butter, lard), fish, fruit, meat, (beef, lamb, pork, game), poultry, and vegetables (including legumes, and vegetable juices) and total grains. Grains consist of many different types of foods, therefore grains were examined as NDSR assigned subgroups rather than as a single combined group (Table 2).
Table 1.
Mean (standard deviation, std) servings per day of major food groups measured at baseline examination year according to personal characteristics in 1101 girlsa: BCERP cohort 2004–2007.
| N | Grains | Dairy | Vegetables | Fruit & fruit juice | Meat including poultry | Fish | Eggs | Fats & oils | Total canned food | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mean (std) | Mean (std) | Mean (std) | Mean (std) | Mean (std) | Mean (std) | Mean (std) | Mean (std) | Mean (std) | |||
|
| |||||||||||
| Age at urine donation (year s) | 6.0 – 6.9 | 277 | 5.42 (1.65) | 1.74 (0.78) | 1.41 (0.8) | 2.05 (1.33) | 2.64 (1.36)* | 0.25 (0.52) | 0.23 (0.33) | 1.92 (1.3) | 0.51 (0.45) |
| 7.0 – 7.9 | 583 | 5.60 (1.71) | 1.79 (0.83) | 1.48 (0.83) | 2.14 (1.41) | 2.60 (1.34) | 0.29 (0.65) | 0.24 (0.33) | 2.04 (1.29) | 0.49 (0.46) | |
| ≥ 8 | 241 | 5.61 (1.68) | 1.85 (0.88) | 1.54 (0.91) | 2.01 (1.32) | 2.93 (1.47) | 0.27 (0.63) | 0.22 (0.29) | 2.06 (1.27) | 0.56 (0.49) | |
|
| |||||||||||
| Race/ethnicity | White | 383 | 5.97 (1.54)* | 2.01 (0.88)* | 1.52 (0.95)* | 1.97 (1.36)* | 2.20 (1.26)* | 0.18 (0.43)* | 0.18 (0.29)* | 2.05 (1.29)* | 0.50 (0.43)* |
| Asian | 56 | 5.68 (1.56) | 1.69 (0.84) | 1.52 (0.72) | 1.87 (1.11) | 2.53 (1.28) | 0.51 (0.91) | 0.28 (0.33) | 2.09 (1.27) | 0.40 (0.36) | |
| Hispanic | 325 | 4.94 (1.79) | 1.81 (0.78) | 1.33 (0.77) | 2.33 (1.35) | 2.62 (1.29) | 0.22 (0.47) | 0.28 (0.35) | 1.78 (1.39) | 0.40 (0.43) | |
| Black | 337 | 5.67 (1.60) | 1.53 (0.74) | 1.55 (0.77) | 2.02 (1.42) | 3.31 (1.37) | 0.40 (0.80) | 0.24 (0.33) | 2.19 (1.15) | 0.64 (0.51) | |
|
| |||||||||||
| BMI- %ile at urine collection | < 50 | 371 | 5.69 (1.75)* | 1.81 (0.90)* | 1.41 (0.79) | 2.01 (1.31) | 2.53 (1.40)* | 0.28 (0.63) | 0.21 (0.30) | 1.94 (1.28)* | 0.48 (0.45) |
| 50 – <85 | 374 | 5.64 (1.74) | 1.86 (0.80) | 1.50 (0.90) | 2.17 (1.32) | 2.67 (1.35) | 0.28 (0.63) | 0.24 (0.33) | 2.16 (1.36) | 0.53 (0.46) | |
| ≥ 85 | 355 | 5.33 (1.54) | 1.70 (0.78) | 1.52 (0.83) | 2.10 (1.47) | 2.85 (1.37) | 0.27 (0.60) | 0.25 (0.34) | 1.95 (1.21) | 0.52 (0.49) | |
|
| |||||||||||
| Caregiver’s education | ≤ HS diploma | 308 | 5.14 (1.84)* | 1.78 (0.77) | 1.32 (0.76)* | 2.17 (1.38) | 2.78 (1.36) | 0.23 (0.56) | 0.24 (0.32) | 1.87 (1.38)* | 0.43 (0.46)* |
| ≥Some college | 766 | 5.73 (1.56) | 1.79 (0.86) | 1.54 (0.87) | 2.06 (1.37) | 2.62 (1.40) | 0.30 (0.64) | 0.23 (0.32) | 2.08 (1.25) | 0.54 (0.46) | |
|
| |||||||||||
| Site | NY | 351 | 4.96 (1.69)* | 1.68 (0.78)* | 1.34 (0.73)* | 2.24 (1.45)* | 2.96 (1.39)* | 0.29 (0.60) | 0.27 (0.34)* | 1.91 (1.26)* | 0.47 (0.48)* |
| OH | 329 | 5.75 (1.53) | 1.95 (0.83) | 1.39 (0.79) | 1.67 (1.20) | 2.79 (1.36) | 0.22 (0.64) | 0.16 (0.26) | 1.93 (1.20) | 0.62 (0.48) | |
| CA | 421 | 5.91 (1.67) | 1.75 (0.86) | 1.66 (0.93) | 2.29 (1.36) | 2.37 (1.32) | 0.31 (0.61) | 0.26 (0.34) | 2.17 (1.36) | 0.45 (0.42) | |
|
| |||||||||||
| All BCERP participants | 1101 | 5.56 (1.69) | 1.79 (0.83) | 1.48 (0.84) | 2.09 (1.37) | 2.68 (1.38) | 0.28 (0.62) | 0.23 (0.32) | 2.0 (1.30) | 0.51 (0.46) | |
|
| |||||||||||
| NHAN ES1 | 215 | 6.66 (3.87) | 2.61 (2.87) | 0.96 (0.47) | 1.14 (1.49) | 2.92 (1.97) | 0.25 (0.72) | 0.27 (0.49) | NC2 | NC2 | |
Table includes all study participants with both dietary recall information and biomarker data
Calculated from What We Eat in America 2003–4 (mean age 7.5 years). www.cdc.gov/nchs/NHANES
NC: Not calculated
ANOVA F test:, p< 0.05
Table 2.
Consumption of major and minor food groups among 1101 BCERP girls (2004–2007).1
| Food groups | Mean (Std) Servings per day |
|---|---|
| Total Grains | 5.56 (1.69) |
|
| |
| Grains, flour and dry mixes2 | 1.14 (1.01) |
| Rice | 0.21 (0.30) |
| Flour in mixed foods | 0.25 (0.33) |
| Bread | 2.26 (1.13) |
| Pasta | 0.58 (0.63) |
| Ready-made cereal | 0.60 (0.54) |
| Cakes, pastries | 0.47 (0.46) |
| Total Dairy | 1.79 (0.83) |
|
| |
| Whole fat dairy | 0.56 (0.52) |
| Low fat dairy | 1.22 (0.76) |
| Total Vegetables | 1.48 (0.84) |
|
| |
| Fresh vegetables | 0.81 (0.65) |
| Non-fresh vegetables | |
| Processed | 0.71 (0.51) |
| Canned | 0.31 (0.35) |
| Total Fruit | 2.09 (1.37) |
|
| |
| Fresh fruit | 0.83 (0.99) |
| Non-Fresh | |
| Processed | 0.66 (0.58) |
| Canned | 0.15 (0.25) |
| Total Meat | 2.68 (1.38) |
|
| |
| Non-poultry meat | 1.50 (1.07) |
| Poultry | 1.18 (0.99) |
| Poultry excluding fried chicken | 0.79 (0.82) |
| Fried chicken: fast food, commercial entrees | 0.39 (0.65) |
| Total fish and shellfish | 0.28 (0.62) |
|
| |
| Fresh | 0.21 (0.51) |
| Non-Fresh | 0.03 (0.12) |
| Canned | 0.03 (0.12) |
| Fried, fast food | 0.04 (0.18) |
| Eggs | 0.23 (0.32) |
|
| |
| Fats and oils | 2.02 (1.29) |
|
| |
| Total canned foods | 0.51 (0.46) |
Includes NDSR food groupings and subgroups created to distinguish food packaging and processing that are used to examine associations with biomarkers.
Average servings per day subgroups do not necessarily add up to the total group as only the main subgroups and foods are shown. Intended subgroups are all or some of the subgroups.
During the dietary recall process, participants describe details about the food preparation. NDSR translates this information into a detailed list of ingredients. We used this list to develop food sub-categories that reflect sources of exposure from food processing and packaging by scanning records (n=4500) for key words that differentiate non-fresh from fresh foods. Non-fresh foods were foods that had key words denoting commercial processing or packaging such as ‘canned’, ‘commercial’, ‘coated’, ‘dehydrated’, ‘fast food’, ‘flavored’, ‘frozen’ and ‘processed’. For example, we categorized kidney beans as fresh when described as ‘vegetables, beans, kidney beans, cooked from dried’ and as non-fresh when described as ‘vegetables, beans, kidney beans, canned –drained, regular’. In some instances where a fresh food was considered to be part of a processed food, it was categorized as non-fresh. For example, the potato component in French fries is described as “vegetables, potato, without skin.” It was necessary to look at the whole food description to correctly categorize as non-fresh. The categorization of fresh and non-fresh foods was done predominantly for vegetables (which included legumes) and fruits, as these foods are available for purchase either fresh or with packaging or processing, whereas foods like meats, dairy, and grain-based food are generally packaged or processed. Additionally we broke down the NDSR variable named ‘Grains, flour, and dry mixes’ as this grouping contained grains which are part of other foods (flour from mixed foods). Utilizing these approaches, we created food sub-groups (Tables 2 and 3) which were used as food predictors in our analyses.
Table 3.
Adjusted1 geometric means (95% CI) of urinary phthalate and phenol biomarkers (μg/gC) in relation to intake of food groups and sub-groups2 in 1101 girls (BCERP cohort 2004–7) for selected items showing associations with biomarkers ( p<0.10).
| FOOD GROUP | BPA (μg/gC) | ||||
|---|---|---|---|---|---|
|
| |||||
| Servings per day | |||||
| 0- < 0.5 | 0.5- <1.0 | ≥ 1.0 | p- trend3 | ||
| Grains, flour and dry mixes | GM (CI) | 2.22 (1.98–2.50) | 2.58 (2.28–2.93) | 2.75 (2.51–3.03) | 0.001 |
| N | 312 | 261 | 501 | ||
| Flour in mixed foods4,5 | GM (CI) | 2.49 (2.29–2.71) | 3.00 (2.57–3.51) | 2.80 (2.17–3.62) | 0.038 |
| N | 890 | 138 | 46 | ||
| Total vegetables | GM (CI) | 2.30 (1.93–2.74) | 2.30 (2.03–2.61) | 2.71 (2.48–2.96) | 0.006 |
| N | 82 | 220 | 772 | ||
| Non-fresh vegetables5 | GM (CI) | 2.36 (2.14–2.62) | 2.68 (2.40–2.99) | 2.81 (2.49–3.18) | 0.010 |
| N | 447 | 352 | 275 | ||
| Total poultry | GM (CI) | 2.36 (2.10–2.65) | 2.58 (2.27–2.93) | 2.70 (2.45–2.97) | 0.043 |
| N | 292 | 253 | 529 | ||
| Poultry excluding fried/fast food5,6 | GM (CI) | 2.41 (2.18,2.67) | 2.54 (2.25,2.87) | 2.84 (2.54,3. 18) | 0.011 |
| N | 479 | 251 | 344 | ||
| Fats | GM (CI) | 2.11 (1.69–2.64) | 2.29 (1.99–2.63) | 2.68 (2.46–2.92) | 0.005 |
| N | 64 | 181 | 829 | ||
| Total fish | GM (CI) | 2.51 (2.30,2.73) | 2.75 (2.21,3.43) | 2.93 (2.48,3. 46) | 0.050 |
| N | 888 | 63 | 123 | ||
| Fried fish, fast food5 | GM (CI) | 2.54 (2.34–2.76) | 3.28 (2.44–4.43) | 3.83 (2.23–6.58) | 0.028 |
| N | 1030 | 34 | 10 | ||
| ΣParabens (μg/gC) | |||||
|---|---|---|---|---|---|
|
| |||||
| Servings per day | |||||
| 0- < 0.5 | 0.5- <1.0 | ≥ 1.0 | p- trend | ||
| Non-fresh vegetables | GM (CI) | 99 (84–117) | 103 (86–123) | 78 (65–96) | 0.046 |
| N | 447 | 352 | 275 | ||
| Poultry excluding fried/fast food6 | GM (CI) | 85 (72,100) | 102 (83,125) | 105 (87,127) | 0.035 |
| N | 479 | 251 | 344 | ||
| ΣDEHP (μg/gC) | |||||
|---|---|---|---|---|---|
|
| |||||
| Servings per day | |||||
| 0- < 0.5 | 0.5- <1.0 | ≥ 1.0 | p- trend | ||
| Grains, flour and dry mixes | GM (CI) | 150 (133–169) | 155 (137–176) | 182 (166–200) | 0.001 |
| N | 312 | 261 | 501 | ||
| Rice5 | GM (CI) | 162 (148–177) | 196 (167–230) | 166 (116–237) | 0.084 |
| N | 924 | 127 | 23 | ||
| Whole Fat Dairy | GM (CI) | 165 (151–181) | 156 (135–173) | 198 (172–228) | 0.051 |
| N | 611 | 277 | 186 | ||
| Total fish | GM (CI) | 164 (150–178) | 157 (126–196) | 205 (173–242) | 0.018 |
| N | 888 | 63 | 123 | ||
| Fried fish, fast food5 | GM (CI) | 165 (152–179) | 231 (171–312) | 267 (154–460) | 0.007 |
| N | 1030 | 34 | 10 | ||
| mBzP(μg/gC) | |||||
|---|---|---|---|---|---|
|
| |||||
| Servings per day | |||||
| 0- < 0.5 | 0.5- <1.0 | ≥ 1.0 | p- trend | ||
| Grains, flour and dry mixes | GM (CI) | 16.2 (11.5–23.0) | 15.5 (12.6–18.8) | 20.6 (18.8–22.7) | 0.003 |
| N | 312 | 261 | 501 | ||
| Flour from mixed foods5 | GM (CI) | 19.1(17.3–21) | 23.0 (19.3–27.4) | 22.5 (16.8–30.1) | 0.040 |
| N | 890 | 138 | 46 | ||
| Fresh Fruit7 | GM (CI) | 21.1 (18.8–23.6) | 19.9 (17.4–22.8) | 17.6 (15.4–20.1) | 0.016 |
| N | 467 | 295 | 312 | ||
| mCPP (μg/gC) | |||||
|---|---|---|---|---|---|
|
| |||||
| Servings per day | |||||
| 0- < 0.5 | 0.5- <1.0 | ≥ 1.0 | p- trend | ||
| Fish: Fresh | GM (CI) | 5.99 (5.56–6.45) | 6.89 (5.64–8.41) | 6.94 (5.82–8.26) | 0.049 |
| N | 930 | 61 | 83 | ||
| LowMWP (μg/gC) | |||||
|---|---|---|---|---|---|
|
| |||||
| Servings per day | |||||
| 0- < 0.5 | 0.5- <1.0 | ≥ 1.0 | p- trend | ||
| Total poultry | GM (CI) | 142(127,160) | 144(127,163) | 166(151,182) | 0.008 |
| N | 292 | 253 | 529 | ||
| Fried/processed chicken | GM (CI) | 146(134,159) | 175(154,200) | 171(147,199) | 0.005 |
| N | 716 | 210 | 148 | ||
Adjusted for age at urine collection, race and caregiver education.
Groups in Table 2.
Trend test using median of each category as continuous variable
Examples are flour in pizza, gravy.
The number of girls by serving size in subgroups may be higher than the number in the total food group as the total food group included other foods, with more or less servings.
Fried/processed chicken showed no association.
The relationship is inverse.
Foods were categorized as 0- <0.5, 0.5 – < 1 and ≥ 1 servings per day (low, medium and high intake) based on reported intake. Our mean daily servings are lower than USDA recommendations; although the highest category (≥ 1 servings per day) approximates the USDA age recommended total daily amounts for several of the food groups. To calculate the proportion that a subgroup contributes to the total food group, as reported in Results and figures, we first calculated the total number of servings/day of a food group for all girls, for example 100.91 servings /day of fish among the 1101 girls. Then we divided the total subgroup servings (for ex. 12.9 servings of fried fish) by the total in the food group (100.91) to obtain the proportion for a subgroup (12.9%). We categorized meal locations into 3 groups: home; school; and restaurant/deli.
To supplement the data collected through the 24 hour dietary recalls, we ascertained additional sources of dietary exposure in the baseline questionnaire. Girls were asked the weekly frequency of consumption of canned beverages and foods, as well as meats or cheeses that came in plastic wrap or containers during the week and month prior to the interview and urine collection. We examined the exposures derived from these questions separately from the 24 hour recall data.
2.2 Urinary biomarker measurements
Baseline urine samples were analyzed at the National Center for Environmental Health at the CDC. Laboratory analytic methods have been published (Kato et al. 2005; Ye et al. 2005; Ye et al. 2006). Urinary conjugates of the target analytes are enzymatically hydrolyzed, concentrated and separated from other urine components by on-line solid phase extraction coupled to high performance liquid chromatography. Quantitation is achieved by isotope dilution tandem mass spectrometry. Limits of detection (LODs) were calculated as three times the standard deviation of near-zero or blank quality control specimens. Analytic quality control and reagent blank samples included in each analytical batch met the CDC reporting guidelines. All specimen collection and storage materials were supplied by the CDC. The CDC laboratory is certified by the Health Care Financing Administration to comply with the requirements set forth in the Clinical Laboratory Improvement Act of 1988. In addition, QC samples from an external pool were incorporated at each site before shipping. As reported, results of these masked specimens were consistent between sites and batches (Wolff et al. 2010). For the 13 phthalate and phenol metabolites included here, the CV’s were less than 10% for 8 biomarkers and 10–20% for 5 analytes (n=101 control pool specimens). Urinary concentrations were obtained for creatinine and nine phthalate metabolites: monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), mono-(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP), mono-isobutyl phthalate (MiBP), mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); four phenols (BPA, n = 1,149; methyl-, butyl-, and propylparabens, n = 1,059). Butyl paraben and MEHP had the highest %<LOD ( 49% and 19%, respectively). We used log-transformed values of the urinary biomarker concentrations to normalize the distributions, and we substituted the value LOD/✓2 for concentrations below the LOD for the statistical analyses. To reduce multiple comparisons and to examine phthalates previously associated with food sources, we combined the phthalate metabolites into three groups based on molar sums that represent similar sources and similar biologic activity; low molecular-weight phthalate metabolites (low MWP: MEP, MBP, and MiBP), high molecular-weight phthalate metabolites (high MWP: MCPP, MECPP, MEHHP, MEOHP, MEHP and MBzP) and the sum of four DEHP metabolites (ΣDEHP: MEHP, MEHHP, MEOHP, MECPP). Similarly, a molar sum of methyl and propyl parabens was created (Σparaben) expressed as propyl paraben (molecular weight 180.2); butyl paraben was excluded because of the high number of <LOD observations. Concentrations are reported corrected for urinary creatinine (μg/g creatinine) to account for urine dilution. The results for ΣDEHP and high MWP were very similar; therefore only the results from ΣDEHP analyses are presented for ease of comparison with other studies.
2.3 Statistical Analysis
Covariate-adjusted least squares geometric means and 95% confidence intervals of creatinine-corrected phthalate and phenol metabolite concentrations in urine in relation to food intake were calculated using PROC GLM in SAS 9.2. Potential confounders were considered as covariates if the bivariate analyses showed differences in both diet and biomarker distributions by confounder category (p < 0.05 pearson’s Chi-square). Caregiver’s education (categorized as high school diploma or less and some college or more) was used as an indicator of socioeconomic status and met the above criteria. Diet and biomarker levels also differed by child’s race/ethnicity and age at urine collection, so these were also included in the final models. Total calories did not meet the definition of true confounder in our data and body mass index percentile (BMI%) was not included as it is a potential collider in causal pathway models (Hernan and Cole 2009). As stated above, the biomarker concentrations are creatinine corrected to reduce misclassification due to different urine dilutions. There were differences in mean servings of certain foods and levels of biomarkers by site, and it is possible that specific food sources or processing differed by site, so we reran the models including site. Models with and without site provided almost identical results. Since we had no specific evidence to show that food sources of these chemicals differed by geographic region, we excluded site as a confounder. Therefore, only results from models without site adjustment are presented, which also allowed us to retain range of diet exposure gained by this multi-site study.
We examined the association between meal location and urinary biomarkers (without foods), adjusted for total meal count per girl. We also examined meal location as a possible covariate in the final model.
2.4 Final Model
Geometric means of urinary biomarkers were adjusted for age, caregiver education, and race/ethnicity. A trend test identified linear associations between food servings groups and urinary metabolites. These were obtained using the median food servings per food category as a continuous variable in the model. Two-sided P value for trend < 0.05 was considered to be statistically significant. We separately examined dietary intake from only the diet call closest to the urine collection, and results were similar to intake from the average of the calls over a year.
3. Results
The population has been described elsewhere (Biro et al. 2010). Girls were 6–8 years old at the time of urine collection; there were similar proportions of White, Black and Hispanic girls. Several baseline characteristics differed by site, as previously reported. Dietary intakes differed by important characteristics including site, BMI% and race (Table 1). There were small differences in diet patterns among the sites, with California girls reporting higher servings of vegetables, grains and fats and the least amount of meat. Cincinnati girls had a higher proportion of canned foods. The mean servings of major food groups were comparable to national data for girls in this age group except for fruit, dairy and grains (Table 1). For most food groups, our data as well as the national means, do not meet the USDA serving recommendations. We calculated means servings for several food subgroups we analyzed (Table 2). In terms of meal location: 78% of the girls’ meals were eaten at home, 11% at school and 7% at either a restaurant or deli (data not shown).
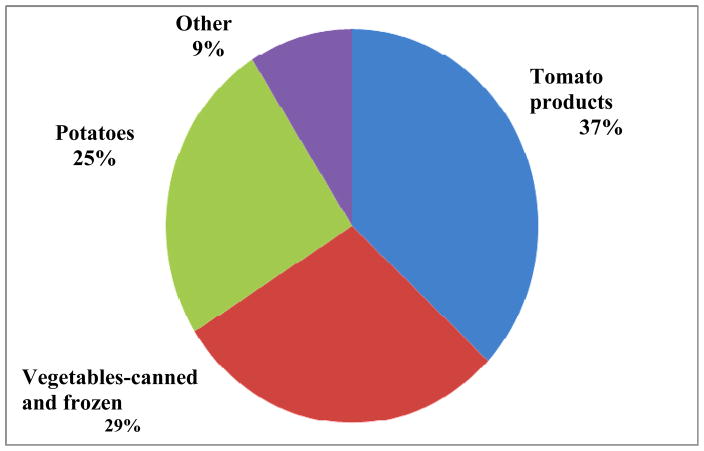
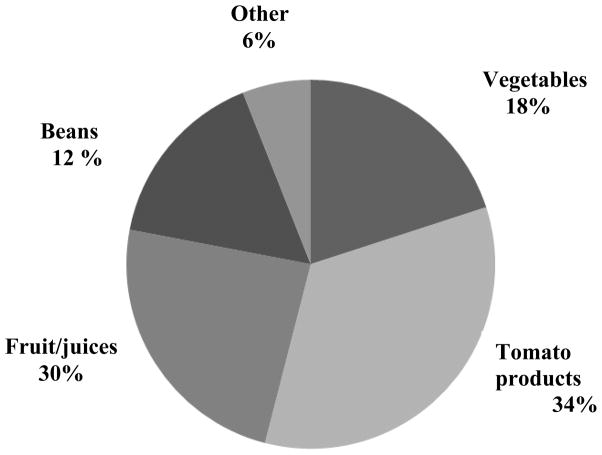
Several food groups were associated with increased urinary concentrations of BPA and ΣDEHP. Increased “grains, flours and dry mixes” (which include rice, oatmeal and flour in mixed foods) and increased total fish consumption were positively associated with BPA and ΣDEHP urinary concentrations. Review of specific types of grains included in this broad category, revealed that “flour in mixed foods” had a strong association with BPA and that rice was associated with ΣDEHP. Rice contributed approximately 35% of grain consumption, and “flour in mixed foods” contributed over 42% of grain consumption. The sub-category, “fried, fast food type fish”, made up 30% of the total fish consumption category and was significantly associated with both BPA and ΣDEHP. Among girls with higher fish consumption (>= 1 serving/day), 18% of the servings were fried, processed/fast food (adjusted geometric mean ΣDEHP 267 μg/gC) compared to only 2.7% of the servings among girls with lower fish consumption (adjusted geometric mean ΣDEHP: 165 μg/gC). Canned fish accounted for 30% of all fish consumption, but when we examined canned fish alone we did not see any associations. Greater total vegetable intake was associated with increased BPA concentration (Table 3). To further examine the relationship between vegetable intake and BPA, we divided the total vegetable servings into two groups; non-fresh (53%) and fresh (47%). Figure 1 shows the items that make up non-fresh vegetables. Non-fresh vegetables were positively associated with BPA and paraben urinary concentrations. We did not see similar associations for non-fresh fruit (44% of total fruit intake). However, we found that fresh fruit was inversely associated with MBzP, a high MWP metabolite, but not with high MWP or ΣDEHP. Figure 2 shows the types of foods that make up canned foods, the majority being tomato-based pasta and pizza sauces, and canned or frozen beans or corn.
Figure 1.
Specific foods and food groups that make up non-fresh vegetable consumption in BCERP cohort 2004–7. Potatoes include french fries, mashed from dehydrated, hash browns and tater tots. Canned and frozen vegetables include: peas, corn, frozen peas, spinach and collards. Tomato products include sauces and pastes from jars and cans. “Other” includes mainly canned beans.
Figure 2.
Specific foods that make up canned good consumption in BCERP cohort 2004–7. “Other” includes fish, meats and gravies.
Fats and oils, which include margarine, oils, shortening, butter and animal fats, were positively associated with BPA. Whole-fat dairy consumption was associated with ΣDEHP. Greater poultry consumption was positively associated with BPA and Σparabens and low MWP (Table 3). Poultry includes fried and processed chicken. The association for low MWP was seen in the total poultry and fried/processed chicken category whereas for BPA and Σparabens, the association was seen for “total chicken”.
No associations were found when we examined meal location alone in relation to biomarkers. When we included the variable meal location in the final model as a covariate (including food variables), associations were similar to the models without it.
We did not find associations of biomarkers with reported intake of canned food and beverages or in plastic packaging, consumed in the prior week and month of the interview, as reported in the baseline questionnaire.
4. Discussion
We found that certain foods predicted higher urinary concentrations of BPA, parabens and ΣDEHP. Our research did not identify exact sources of contamination nor when it may have occurred in the food chain, but the associations we observed are consistent with reports in the literature that link these chemicals to food processing and packaging, and possibly to indirect sources such as cattle feed (Jarosova A 2010). Association of BPA with non-fresh vegetables is reported by others (Braun et al. 2011), including detection of BPA in canned foods (Cao et al. 2010; Kang and Kondo 2002; Schecter et al. 2010). A recent risk assessment suggests that canned vegetables and canned fruits have different contributions to total BPA; canned vegetables contribute 10–40% of the daily BPA intake, whereas canned fruits contribute 3–6% (von et al. 2010). Our results for these two food groups are in line with this and other studies (Braun et al. 2011). Noonan et al. (2011) showed that BPA concentrations in canned foods vary greatly not only between food types but also within food types and between production lots from the same manufacturer. Several factors influence the amount of migration of BPA into canned goods, including the matrix, heat during the sterilization process and the specific kind of can coating. However the extent that these factors determine migration into cans has not been quantified (Grumetto et al. 2008). Parabens in our population were associated with poultry; however they were not related to some foods such as sugary items, marmalades, jellies, baked goods and processed fruits, as had been reported by others (Saad et al. 2005; Soni et al. 2002).
We found relationships between grains, dairy and fish and ΣDEHP, whereas others found associations between poultry or vegetables and ΣDEHP using NHANES data (Colacino et al. 2010). They also found associations with low MWPs and fruits, vegetables and meats. We did not see the latter possibly because our cohort is not the same as NHANES with regards to race, age and geographic location. Additional reasons for differing results could be study design, different dietary assessments, serving cutpoints, changes in packaging over time and foods not being a substantial source of LMW phthalates. Colacino’s study was cross-sectional using one recall the day before urine was collected whereas our dietary data were collected 1–12 months after urine collection. We used dietary servings in 3 categories for each food group whereas Colacino et al. used dietary servings as a continuous variable. Dietary intake measurements attempt to preserve relative rankings rather than to provide accurate continuous variables (Willett 1998). Therefore use of categories (low, medium and high) of food consumption is a more conservative approach than a linear model.
Our association between whole fat dairy consumption and ΣDEHP is supported by studies that have reported similar relationships and that have detected phthalates in dairy products (Colacino et al. 2010; Petersen and Jensen 2010; Sharman et al. 1994). Aluminum paper-foil laminate of butter packaging and dairy tubing are cited as possible sources of DEHP (Sharman et al. 1994). Other foods have also been found to be related to higher DEHP metabolites concentrations, including grains, poultry and fish (Colacino et al. 2010; Fromme et al. 2007a; Lakind and Naiman 2011; Schecter et al. 2013). Two studies that performed quantitative comparison of dietary intake based on duplicate samples with imputed intake from excreted DEHP showed that food is the dominant intake source of DEHP (Fromme et al. 2007; Wormuth et al. 2006) and important sources of dibutyl phthalate and BBP (Wormuth et al. 2006). In contrast, diet accounts for a small fraction of exposure to low MWPs (dimethyl phthalate and diethyl phthalate) which are predominantly used in personal products, such as shampoo (Wormuth et al. 2006). Nevertheless, we and others find associations of low MWPs with certain foods, such as poultry and vegetables (Colacino et al. 2010) and decreased after a fresh food intervention (Rudel et al. 2011).
A number of other studies report food packaging and BPA associations, including carry-out food wrapping (Vandenberg et al. 2007), cans and microwave containers (Carwile et al. 2009; Mariscal-Arcas et al. 2009), and canned tuna fish (Munguia-Lopez et al. 2005). Packaging is thought to be the primary source of phthalate contamination of foods as well. An intervention study in families showed BPA and DEHP exposures substantially reduced when diets were restricted to foods with limited packaging (Rudel et al. 2011). We did not see any associations of urinary biomarkers related to consumption of foods likely to come into contact with these types of packaging when using our self-report questionnaire data. Retrospective and concurrent self-report questions of packaging and canned consumption have the potential to be imprecise from inaccuracies in memory or estimation, which could lead to non-differential misclassification, biasing the results towards the null. Recency has been found to influence children’s recall accuracy; a study found that shortening the retention interval of dietary recalls increases accuracy for reporting energy and macronutrients (Baxter et al. 2004).
Our results suggest that foods like grains and poultry increase urinary concentrations of metabolites of the high and low MWPs, while meat consumption does not. The fact that we don’t see an association with meat is somewhat surprising since parent phthalate diesters are lipophilic and are released from packaging mainly into foods containing fat, such as meats and chicken but not grains. Therefore, there may be alternative routes of food contamination by phthalate diesters for chicken and grains. Colacino also saw differences in associations for chicken and meat (Colacino et al. 2010). Reports show that agricultural crops and animals have contact with these chemicals during cultivation, and such activities probably differ for chicken from meat-producing animals. The NDSR “grains, flour and dry mixes” category includes foods that are not exclusively composed of grains. For example, flour used in pizza is attributed to this category, however pizza also contains other ingredients such as tomato sauce that has more opportunities for contamination during processing and packaging. Pizza’s tomato sauce would be assigned to another food group. This highlights the complexity of the NDSR processing of reported foods and in terms of the data structure, we cannot disentangle exposure sources from each of the ingredients in the food. It was somewhat unexpected that we did not find associations with grain specific groups, such as cookies, pastries and breads. Chemical analysis of foods before and after they reach the market shelves would be one step to understand the points of contamination of our food supply. Colacino et al. suggest consultation from the food industry to help determine what step in the production process contamination is occurring (Colacino et al. 2010).
Our null results when examining meal location differ from Lakind et al. who finds a positive association between BPA and meals not prepared at home and school lunches per week in a population sample (NHANES). One main difference is that our meal location variable does not specify where the meal was prepared, so when a child reports eating at school, we can not differentiate whether it is a school lunch or a meal prepared at home.
There are several limitations to this study. Our study population was recruited from certain groups within 3 geographic locations with most girls residing in either a city or suburban location. Therefore our results are not representative of all US girls. The majority of the girls were born in the United States; however some of their parents were not. Although different dietary habits exist, including purchasing and cooking between first and second generation (Liu et al. 2012) we did not have the data available to analyze this. We used only one sample to characterize each girl’s urinary biomarker level. Because phenol and phthalate metabolites are relatively shortlived in the body, a single sample may not represent the temporal window of exposure relevant to outcomes. An increasing number of reports now document intraindividual variability of urinary phenols and phthalate metabolites over time, among pregnant women (Braun et al. 2011) and adults, including men and women (Fromme et al. 2007; Hauser et al. 2004; Hoppin et al. 2002; Peck et al. 2010), and families including children (Ackerman et al. 2014). Before undertaking this research we conducted a study to establish the reliability of such measurements in children and found that intraindividual variability in children was acceptable over about 12 months (Teitelbaum et al. 2008). Study designs report varied intervals between urine collections, ranging from days to years; studies collect spot samples at various times of day, and they differ with regard to age and sex of subjects. ICC’s (intraclass correlation coefficients) suggest poor to good reliability of a single urine measurement for phthalates (fair-good; ICC ~0.2–0.8), parabens (fair; ICC 0.3–0.6; Meeker; Smith), and BPA (poor; ICC 0.1–0.2) in various reports. However, several studies indicate that ranked exposure categories (e.g. tertiles of urinary biomarkers) are quite consistent over time even when the ICCs are poor (Hauser et al. 2004; Peck et al. 2010). Some phthalate metabolites show better agreement over time (Fromme et al. 2007; Hauser et al. 2004; Hoppin et al. 2002) than others (Ackerman et al. 2014; Fromme et al. 2007; Peck et al. 2010; Preau, Jr. et al. 2010). In the case of this paper, dietary data were collected 2–18 months after the urine specimen. As a further check on temporality of urine measures and diet in this paper, when we limited the dietary data to the call closest to the urine collection, the findings were not improved. The dietary intake method used is primarily designed to gather nutritional information, not to specifically provide the differentiation between fresh and non-fresh foods (processed and packaged). This limited our ability to identify sources of exposure for certain food groups. Lastly, diet alone cannot explain the total source of exposure as there are additional sources of phthalates, BPA and parabens, such as air pollution, personal care products, floor coverings in housing and medications, and these were not considered. We have obtained a detailed inventory of the girls’ exposure to many of these sources and this analysis is the subject of future publication. Together with the dietary data, this information will give us a more complete picture of the total body burden of these chemicals.
Our study has several strengths. Dietary data were the average of 2–4 calls within a year, which has shown to be a reliable measure of usual dietary patterns, including seasonal variation. The very detailed NDSR information allowed us to create a more thorough description of food source (e.g., canned, processed, fresh). Finally, our sample size is relatively large and comprises a diverse group of girls with variable exposures and food habits.
EDs interfere with hormone action, potentially affecting a wide range of health effects, and food is a significant contributor to human ED exposure. Better information on identifying when and how contamination occurs in the food chain may enable children to maintain a healthy diet with less concern about adverse exposure.
Highlights.
BPA and phthalates are found in a wide range of foods and packaging.
Consistent with others, whole fat dairy products were associated ΣDEHP
Grains, fish, non-fresh vegetables, poultry and fats were associated with BPA.
Grains, fish were also associated with ΣDEHP concentrations.
Foods contribute to children’s exposures to certain chemicals
Acknowledgments
The authors wish to thank the study investigators and staff involved in this research including Julie Britton, Ana Mejia, Arkeyris Richiez, Jessica Montana, Rochelle Osborne, Eunpa Chae, Senaka Peter, Lisa Boguski, Barbara Brenner, Gayle Greenberg, Bob Bornschein, Robert Hiatt, Louise Greenspan and Julie Deardorff as well as Ella Samandar, Jim Preau and Jack Reidy at the Centers for Disease Control and Prevention (CDC) for the phthalate metabolites measures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman JM, Dodson RE, Engel CL, Gray JM, Rudel RA. Temporal variability of urinary di(2-ethylhexyl) phthalate metabolites. J Expo Sci Environ Epidemiol. 2014:10. doi: 10.1038/jes.2013.93. [DOI] [PubMed] [Google Scholar]
- Andersen FA, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, et al. Final report of the Cosmetic Ingredient Review Expert Panel amended safety assessment of Calendula officinalis-derived cosmetic ingredients. Int J Toxicol. 2010;29:221S–2243. doi: 10.1177/1091581810384883. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, Foster PM. Pathogenesis of male reproductive tract lesions from gestation through. Toxicol Pathol. 2003;31:397–410. doi: 10.1080/01926230390202335. [DOI] [PubMed] [Google Scholar]
- Baxter SD, Smith AF, Litaker MS, Guinn CH, Shaffer NM, Baglio ML, et al. Recency affects reporting accuracy of children’s dietary recalls. Ann Epidemiol. 2004;14:385–390. doi: 10.1016/j.annepidem.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, et al. Pubertal Assessment Method and Baseline Characteristics in a Mixed Longitudinal Study of Girls. Pediatrics. 2010 doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119:131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect. 2010;118:679–685. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XL, Corriveau J, Popovic S. Bisphenol a in canned food products from canadian markets. J Food Prot. 2010;73:1085–1089. doi: 10.4315/0362-028x-73.6.1085. [DOI] [PubMed] [Google Scholar]
- Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, et al. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117:1368–1372. doi: 10.1289/ehp.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. 2009. [PubMed] [Google Scholar]
- Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect. 2010;118:998–1003. doi: 10.1289/ehp.0901712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008;28:561–578. doi: 10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Gruber L, Schlummer M, Wolz G, Bohmer S, Angerer J, et al. Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ Int. 2007a;33:1012–1020. doi: 10.1016/j.envint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Grumetto L, Montesano D, Seccia S, Albrizio S, Barbato F. Determination of bisphenol a and bisphenol B residues in canned peeled tomatoes by reversed-phase liquid chromatography. J Agric Food Chem. 2008;56:10633–10637. doi: 10.1021/jf802297z. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, Cole SR. Invited Commentary: Causal diagrams and measurement bias. Am J Epidemiol. 2009;170:959–962. doi: 10.1093/aje/kwp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine. Environ Health Perspect. 2002a;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosova AHJKL. Screening of phthalic acid esters in raw materials, premixes and feed additives. Environ Chem Letter. 2010:387–391. [Google Scholar]
- Kang JH, Kondo F. Bisphenol A migration from cans containing coffee and caffeine. Food Addit Contam. 2002;19:886–890. doi: 10.1080/02652030210147278. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2011;21:272–279. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De FC, Presta G, Del VA, Paris I, Ruggieri F, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human. Environ Health Perspect. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Chu YH, Frongillo EA, Probst JC. Generation and acculturation status are associated with dietary intake. J Nutr. 2012;142:298–305. doi: 10.3945/jn.111.145516. [DOI] [PubMed] [Google Scholar]
- Mariscal-Arcas M, Rivas A, Granada A, Monteagudo C, Murcia MA, Olea-Serrano F. Dietary exposure assessment of pregnant women to bisphenol-A from cans and microwave containers in Southern Spain. Food Chem Toxicol. 2009;47:506–510. doi: 10.1016/j.fct.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect. 2011;119:252–257. doi: 10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munguia-Lopez EM, Gerardo-Lugo S, Peralta E, Bolumen S, Soto-Valdez H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit Contam. 2005;22:892–898. doi: 10.1080/02652030500163674. [DOI] [PubMed] [Google Scholar]
- Peck JD, Sweeney AM, Symanski E, Gardiner J, Silva MJ, Calafat AM, et al. Intra- and inter-individual variability of urinary phthalate metabolite concentrations in Hmong women of reproductive age. J Expo Sci Environ Epidemiol. 2010;20:90–100. doi: 10.1038/jes.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JH, Jensen LK. Phthalates and food-contact materials: enforcing the 2008 European Union plastics legislation. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27:1608–1616. doi: 10.1080/19440049.2010.501825. [DOI] [PubMed] [Google Scholar]
- Preau JL, Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of. Environ Health Perspect. 2010;118:1748–1754. doi: 10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad B, Bari MF, Saleh MI, Ahmad K, Talib MK. Simultaneous determination of preservatives (benzoic acid, sorbic acid, methylparaben and propylparaben) in foodstuffs using high-performance liquid chromatography. J Chromatogr A. 2005;1073:393–397. doi: 10.1016/j.chroma.2004.10.105. [DOI] [PubMed] [Google Scholar]
- Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, Bingham S, et al. A comparison of a food frequency questionnaire with a 24-hour recall. Int J Epidemiol. 2003;32:1054–1062. doi: 10.1093/ije/dyg264. [DOI] [PubMed] [Google Scholar]
- Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, et al. Phthalate Concentrations and Dietary Exposure from Food Purchased in New York State. Environ Health Perspect. 2013 doi: 10.1289/ehp.1206367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Malik N, Haffner D, Smith S, Harris TR, Paepke O, et al. Bisphenol A (BPA) in U.S. food. Environ Sci Technol. 2010;44:9425–9430. doi: 10.1021/es102785d. [DOI] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Sharman M, Read WA, Castle L, Gilbert J. Levels of di-(2-ethylhexyl)phthalate and total phthalate esters in milk, cream, butter and cheese. Food Addit Contam. 1994;11:375–385. doi: 10.1080/02652039409374236. [DOI] [PubMed] [Google Scholar]
- Soni MG, Burdock GA, Taylor SL, Greenberg NA. Safety assessment of propyl paraben: a review of the published literature. Food Chem Toxicol. 2001;39:513–532. doi: 10.1016/s0278-6915(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol. 2002;40:1335–1373. doi: 10.1016/s0278-6915(02)00107-2. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Trasande L, Sathyanarayana S, Jo MM, Gross S, Attina TM, Mendelsohn AL. Phthalates and the diets of US children and adolescents. Environ Res. 2013;126:84–90. 84–90. doi: 10.1016/j.envres.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Tsumura Y, Ishimitsu S, Saito I, Sakai H, Kobayashi Y, Tonogai Y. Eleven phthalate esters and di(2-ethylhexyl) adipate in one-week duplicate diet samples obtained from hospitals and their estimated daily intake. Food Addit Contam. 2001;18:449–460. doi: 10.1080/02652030117484. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture ARS2. USDA-ARS; 2010. Available: http://www.ars.usda.gov/Services/docs.htm?docid=17558. [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- von GN, Wormuth M, Scheringer M, Hungerbuhler K. Bisphenol a: how the most relevant exposure sources contribute to total consumer exposure. Risk Anal. 2010;30:473–487. doi: 10.1111/j.1539-6924.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. 2. New York City: Oxford University Press; 1998. [Google Scholar]
- Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect. 2010;118:1039–1046. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:53–59. doi: 10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]