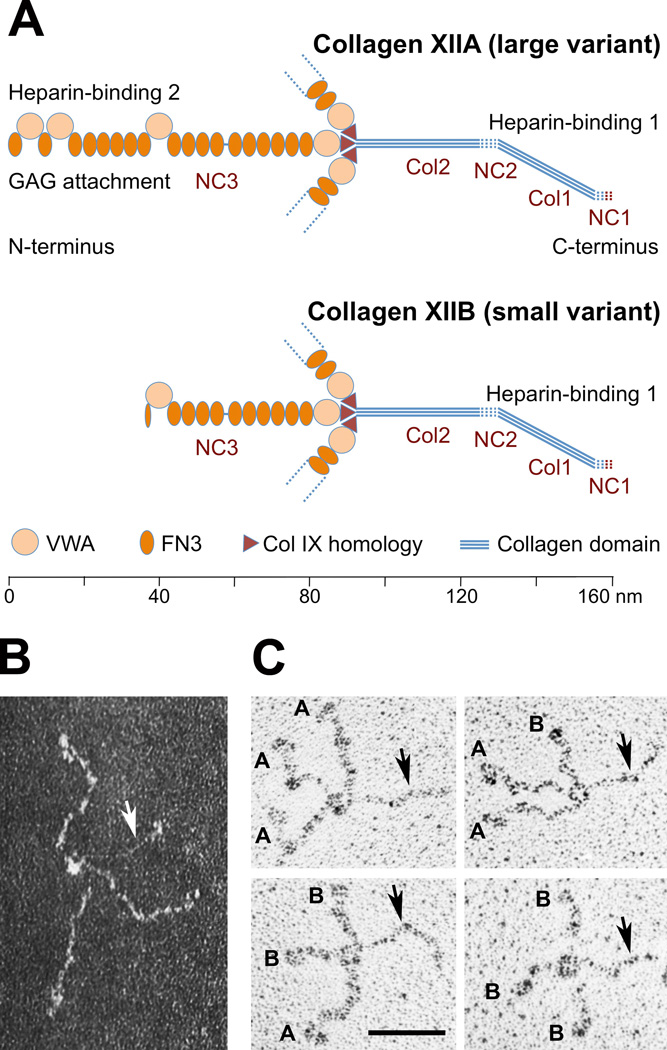
Fig. 1. Structure of collagen XII.
(A) Domain structure of "large" collagen XIIA and "small" collagen XIIB splice variants. Collagen XIIB misses the entire N-terminal half of the NC3 domain. The alternatively spliced region (8 FN3 and 2 VWA modules) contains sites for heparin-binding and glycosaminoclycan (GAG) attachment. A second heparin-binding site is present in the collagenous domain. (B) A collagen XII molecule with three XIIA subunits as seen in the electron microscope after negative staining. Note three flexible arms ≈90 nm in length corresponding to the NC3 domains, along which globular VWA modules are visible. A thin, ≈70 nm long stiff rod with a kink (arrow), the collagen triple helix, emanates from the central globe connecting NC3 domains. (C) Homo- and heterotrimeric collagen XIIA/XIIB molecules, isolated by immuno-affinity chromatography, as seen after rotary shadowing. XIIA and XIIB subunits of individual molecules were identified from the length of their NC3 domains (≈90 and ≈50 nm, respectively; Watt et al. [1992], Koch et al. [1994]). Images (from top left to bottom right) show collagen XIIA3, A2B, AB2, and B3, respectively. NC3 domains are labeled with A or B; collagen domains with arrows. Bar, 50 nm. (A, C) Modified and reproduced from Koch et al. (1994) and (B) from Koch et al. (1992).