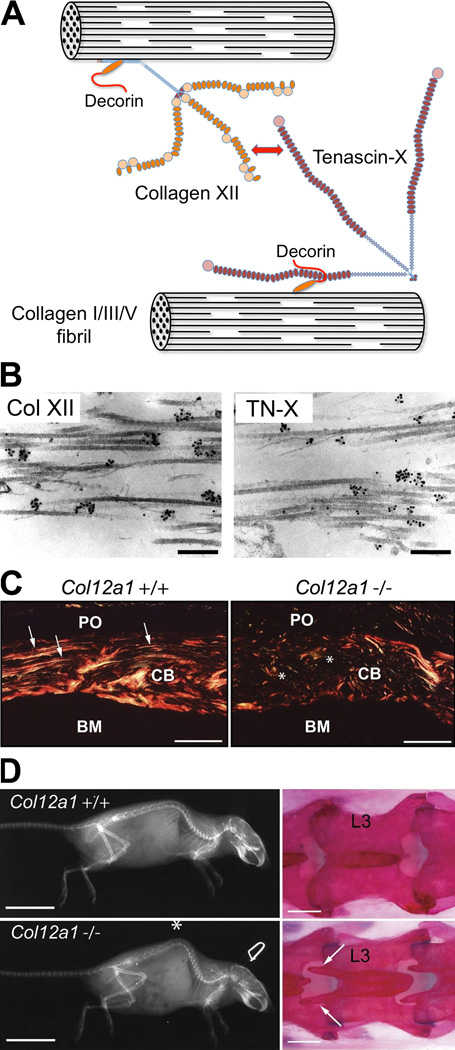
Fig. 2. Function of collagen XII.
(A) Cross-bridging of collagen I-containing fibrils by collagen XII and tenascin-X. Collagen XII interacts with the fibril surface via its collagenous domain; this might be facilitated by a ternary complex with small proteoglycan decorin. NC3 domains of collagen XII extend from the fibril surface and bind tenascin-X, a trimer of subunits with EGF repeats (blue), FN3 domains (purple), and a C-terminal fibrinogen-like globe (pink). Like collagen XII, tenascin-X binds decorin that links it to fibrils. (B) Ultrastructural colocalization of collagen XII and tenascin-X in embryonic dermis. Ultrathin sections were incubated with polyclonal antibodies to collagen XII (left) or tenascin-X (right) followed by colloidal gold-labeled secondary antibody. Note clusters of gold particles associated with cross-striated collagen fibrils in both images. Reproduced from Veit et al. (2006). (C) Disorganized collagen fiber arrangement in bone matrix of collagen XII-deficient mice. Collagen organization was analyzed in femurs of P14 Col12a1+/+ and Col12a1−/− mice under polarized light after Sirius red staining. PO, periosteum; CB, cortical bone, BM, bone marrow. (D) X-ray (left) and alizarin red staining (right) of adult Col12a1+/+ and Col12a1−/− mice. Note kyphosis of the spine (asterisk) and abnormal spinous processes of lumbar vertebrae (marked on vertebra L3 with arrows) in the Col12a1−/− mouse. (A, B) Modified and reproduced from Veit et al. (2006). (C, D) Reproduced from Izu et al. (2011). Bars: 50 nm (B); 50 µm (C); 2 cm (D, left); 1 mm (D, right).