Abstract
Infection by Leishmania takes place in the context of inflammation and tissue repair. Besides tissue resident macrophages, inflammatory macrophages and neutrophils are recruited to the infection site and serve both as host cells and as effectors against infection. Recent studies suggest additional important roles for monocytes and dendritic cells. This paper addresses recent experimental findings regarding the regulation of Leishmania major infection by these major phagocyte populations. In addition, the role of IL-4 on dendritic cells and monocytes is discussed.
1. Introduction
Infection of humans with Leishmania surmounts 1.3 million new cases each year. Parasites infect and survive within phagolysosomal vesicles in host macrophages. Following infection with Leishmania, macrophages produce reactive oxygen species (ROS), cytokines, and chemokines and recruit an early inflammatory reaction [1, 2]. Interactions with inflammatory neutrophils either increase or decrease L. major replication in macrophages depending on host genotype and through mechanisms involving TGF-β or neutrophil elastase [3–5]. Recent studies suggest that additional phagocytes such as monocytes and dendritic cells (DCs) play important roles in infection, both as host and as effector cells. Here we discuss recent experimental findings regarding regulation of L. major infection by these major phagocyte populations. In addition, the role of IL-4 on DC and monocyte responses to infection is discussed.
2. Macrophage Activation
In response to microbial stimulation, macrophages differentiate into distinct M1 and M2 phenotypes [6, 7]. Both M1 and M2 macrophages are induced in the course of Leishmania infection. Microbial stimulation following priming with the Th1 cytokine IFN-γ leads to classically activated or M1-type macrophages [6]. M1 macrophages express nitric oxide (NO)-dependent leishmanicidal activity and are important for control of Leishmania infection [7]. By contrast, M2-type macrophages, induced by the Th2 cytokine IL-4, express arginase and play an important role in tissue repair [6]. Expression of PPAR-γ is also required for induction of M2 macrophages [8]. Mice lacking M2 or alternatively activated macrophages due to genetic deficiency of either the IL-4 receptor or PPAR-γ are more resistant to L. major infection [8, 9]. In addition, host IgG promotes infection by Leishmania due to early effects on macrophage differentiation [10]. Ligation of Fcγ receptors by IgG immune complexes induces IL-10 production and imprints a regulatory or M2b phenotype in macrophages, which is permissive for Leishmania replication [7, 11]. These results suggest that L. major takes advantage of M2 macrophages to replicate in the host.
Infection by L. major takes place in the context of inflammation and tissue repair induced by the insect bite [12–14]. Insect salivary molecules play an important role in the establishment of infection. Maxadilan, an insect derived salivary peptide, modulates the immune response of the host and reprograms dendritic cell maturation to facilitate infection [15, 16]. In addition, molecular and cellular elements recruited by the inflammatory reaction modulate macrophage/Leishmania interactions. Tissue repair is a conserved response to injury characterized by an initial influx of neutrophils, followed by monocyte/macrophages and fibroblasts. Repair cannot be completed until inflammation is resolved, and, in the case of L. major infection, chronic ulcers are associated with persisting infiltrates of neutrophils [13]. Dynamic intravital microscopy of L. major infection site indicates that, after 1 day of infection, parasites localize mainly inside neutrophils [14]. Later, neutrophils are cleared and parasites become localized to monocyte/macrophages [14]. Interestingly, viable parasites are released from apoptotic neutrophils in the vicinity of macrophages [14]. Therefore, it is possible that Leishmania hides in neutrophils until apoptosis forces the parasites to infect a macrophage.
3. Early Macrophage Responses following Infection with L. major
Infection of resident macrophages plays an important sentinel role in early stages of Leishmania infection. Macrophages respond to infectious stress by either adapting or undergoing apoptosis. We investigated the role of cellular stress and apoptosis in macrophages infected with L. major in both susceptible and resistant mice. FasL plays a major role in early responses of susceptible BALB/c mice to infection. Infection induces FasL-dependent apoptosis of Mac-1/CD11bhi resident macrophages, concomitant with secretion of chemokines KC and MIP-1α, and neutrophil extravasation [4]. Apoptosis and chemokine secretion induced by L. major in resident macrophages can be prevented with a neutralizing antibody specific for FasL, and neutrophil extravasation is reduced in FasL-deficient gld mutant mice [4]. These results agree with studies showing resident macrophage demise, chemokine secretion, and neutrophil extravasation following FasL stimulation of macrophages [17].
In contrast, early responses of resistant mice to infection are independent of FasL. Macrophages from B6 mice do not undergo apoptosis upon L. major infection. Chemokine secretion is independent of FasL expression, and neutrophil extravasation induced by infection is preserved in FasL-deficient mice [18]. In addition, we found no sign of macrophage death, and infected B6 macrophages remained viable as judged by constitutive secretion of lysozyme [18]. The reason for the different role of FasL in these mouse strains is unknown. However, BALB/c and B6 mice express a genetic polymorphism in FasL that affects biological activity, and B6 FasL has less cytotoxic activity than BALB FasL [19].
Although infection with Leishmania fails to induce apoptosis, it induces a cellular stress response in resident B6 macrophages characterized by increased production of ROS, activation of the stress activated protein kinases JNK, activation of c-Jun, and increased expression of FasL in resident macrophages [18]. Infection increased secretion of cytokines/chemokines TNF-α, IL-6, TIMP-1, IL-1RA, G-CSF, TREM, KC, MIP-1α, MIP-1β, MCP-1, and MIP-2 in resident macrophages. Secretion of KC is blocked either by addition of antioxidants or by a JNK inhibitor, suggesting that the stress response is involved in chemokine secretion. Interestingly, antioxidants and JNK inhibitor also blocked the intracellular growth of parasites [18], although the mechanisms involved remain to be determined. These results suggest that a cellular stress response by resident B6 macrophages recruits inflammatory cells but also promotes the intracellular survival/growth of the parasite [18].
4. Infection with L. major Interferes with Cytokine-Induced Macrophage Differentiation
In response to cytokines, macrophages differentiate to effector functions. Macrophages treated with a combination of IFN-γ plus IL-4 express leishmanicidal activity [20] and, following restimulation with LPS, secrete nitrites and IL-12, two markers of M1 differentiation [21]. We investigated the effect of prior Leishmania infection on cytokine induced macrophage differentiation. Macrophages treated with IFN-γ plus IL-4 produced increased levels of NO and IL-12p40 following restimulation with LPS (Figure 1(a)). Prior infection with L. major substantially reduced the ability to produce NO and IL-12p40 (Figure 1(a)). Prior infection did not reduce TNF-α response (Figure 1(a)), arguing against a defect in the response to LPS and suggesting a specific defect in IL-12 and nitrite secretion. We also investigated expression of LIGHT, a marker of M2b macrophage differentiation [7]. Prior infection with L. major increased expression of LIGHT in macrophages treated with IFN-γ plus IL-4 (Figure 1(b)). Therefore, prior infection with L. major reduces expression of M1 differentiation markers, whereas it increases expression of M2 differentiation marker. Interestingly, infection with L. major after treatment with IFN-γ did not modulate expression of differentiation markers (results not shown). Together, these data suggest that L. major modulates macrophage differentiation to ensure its intracellular survival. Interestingly, production of ROS is required for inducing M2, but not M1 macrophage differentiation [22], suggesting that the initial stress response could be involved.
Figure 1.
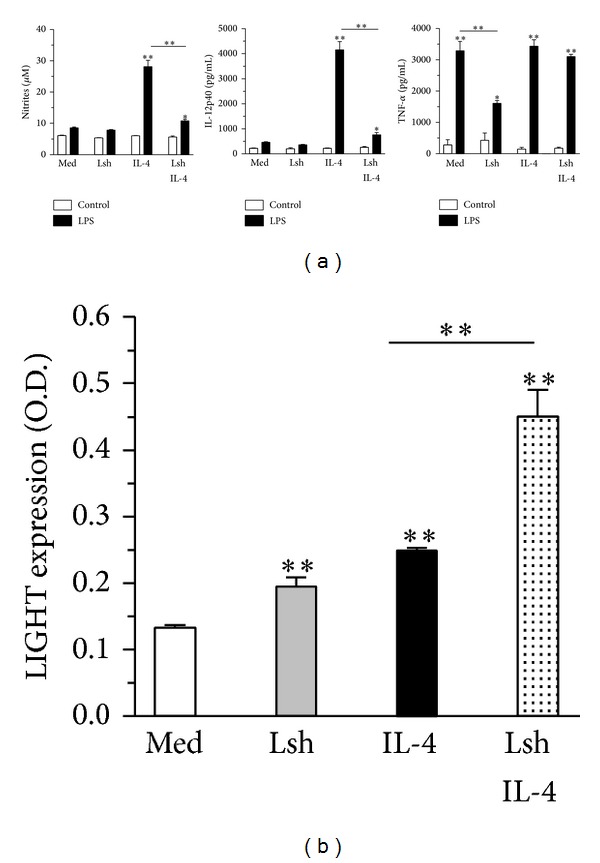
Infection with L. major reduces production of NO and IL-12 and increases expression of M2 marker LIGHT by differentiated macrophages. (a-b) Bone marrow derived macrophages (BMDM from B6 mice) were infected or not with L. major after 6 days. Next day, all BMDM were treated with IFN-γ, and next day, some of the BMDM cultures were treated with IL-4. (a) After 3 d, cells were washed and restimulated with medium (control) or LPS. After 2 additional days, the levels of nitrites, IL-12p40, and TNF-α were determined. (b) After 3 d, expression of LIGHT was evaluated by cellular ELISA. Results are expressed as mean and SE of triplicates (a) or quadruplicates (b), *P < 0.05, **P < 0.01.
5. Engulfment of Neutrophils Regulates Infection of Macrophages
Neutrophils play important roles in the innate immune response. Neutrophils are short lived cells that undergo spontaneous apoptosis following transmigration of blood vessels [23]. Neutrophil apoptosis induces phagocytic clearance and secretion of cytokines by macrophages, which are important for resolution of inflammation and tissue repair [23]. Interestingly, different outcomes of L. major infection and cytokine production are observed in macrophages engulfing apoptotic neutrophils, depending on host genetic background [3]. Engulfment of apoptotic BALB/c neutrophils induces production of TGF-β, but not TNF-α in macrophages, and increases growth of L. major in a manner dependent on PGE2 and TGF-β [3]. Depletion of neutrophils with anti-Gr1 antibody reduces, and adoptive transfer of apoptotic neutrophils increases infection in lymph nodes of BALB/c mice, suggesting a disease promoting role of neutrophils [3]. On the other hand, engulfment of B6 neutrophils induces production of TNF-α, and not TGF-β, in infected macrophages and reduces L. major intramacrophagic load in a manner dependent on TNF-α [3]. Depletion of neutrophils with anti-Gr1 antibody increases, and adoptive transfer of apoptotic neutrophils decreases infection in lymph nodes of B6 mice, role. Neutralization of neutrophil elastase (NE) with a specific inhibitor peptide abrogates the inflammatory clearance of B6 neutrophils and increases infection in lymph nodes [3].
6. Macrophage Activation and Differentiation Induced by Neutrophil Engulfment and Neutrophil Elastase
Clearance of apoptotic cells can be proinflammatory in the presence of additional innate immune stimuli, such as TLR ligands [24, 25]. This observation helps to explain the puzzling proinflammatory effects of phagocytosis of apoptotic B6 neutrophils. NE triggers proinflammatory responses through TLR4 [26, 27]. Purified NE triggers TNF-α production by macrophages and induces leishmanicidal activity in a manner dependent on TLR4 [5]. Interestingly, B6 neutrophils release 2-3-fold more NE than BALB/c neutrophils. In addition, mutant pallid B6 neutrophils, which fail to release NE, do not induce killing of L. major [5]. These results suggest that the proinflammatory and leishmanicidal activities of B6 neutrophils are due to efficient amounts of released NE and perhaps other released granule proteins.
One important question is whether neutrophil engulfment imprints a particular phenotype in macrophages. Our results indicate that proinflammatory, but not antiinflammatory clearance of neutrophils induces a sustained regulatory or M2b phenotype in macrophages, characterized by low IL-12p70 and high IL-10 production following restimulation with LPS, increased expression of LIGHT, induction of Th2 responses, and permissive replication of L. major [21]. As expected, the ability of senescent neutrophils to induce the regulatory phenotype requires NE activity and TLR4 expression [21]. Moreover, previous injection of senescent neutrophils enhances subsequent infection in vivo [21]. These results suggest that induction of regulatory macrophages plays a permissive role in establishment of infection.
7. Monocytes and DCs as Phagocytes and Effector Cells
Monocytes are crucial to immunity against L. major infection as precursors of macrophages and inflammatory DCs. On the other hand, the role of monocytes as key effectors of parasite killing remains elusive, since current monocyte depletion models fail to spare the other cell types. Accordingly, CCR2 is required for mobilization of monocytes from bone marrow [28] and for effective control of L. major infection [29–31]. Whether CCR2-dependent immunity to L. major relies on direct effector role of monocytes, in the generation of effector macrophages/DCs, or both is still debatable [29–32]. Conversely, attempts to deplete only neutrophils by using antibody against Gr1, which encompasses both Ly6C (monocyte) and Ly6G (neutrophil) epitopes, more likely result in monocyte depletion as well.
Infection with L. major increases both myelopoiesis and the number of circulating myeloid precursors [33]. In addition, purified blood monocytes are already able to phagocytose L. major parasites [34]. Blood monocytes, however, may not match the activation state of elicited monocytes in the infection site [34, 35]. More important, different degrees of monocyte maturation, either in the bone marrow or in the infection site, might confer susceptibility or resistance to L. major infection in BALB/c versus B6 mice [13, 36, 37].
Immature myeloid cells were once considered as “safe targets” for Leishmania infection [33, 38]. Indeed, immature macrophages expressing myeloid markers were found as major parasite reservoirs in skin lesions of BALB/c mice infected with L. major even 4 weeks after infection [13, 36, 38]. Recent evidences indicate that monocytes/macrophages predominate as infected cells at early (3 days) and late (4 wks) time points in skin lesions, whereas infected DCs accumulate at 4 weeks in lesions and are always dominant in B6 or BALB/c draining lymph nodes [32, 39]. In spite of differences in phagocyte populations in different sites and routes of infection [40], detailed analyses in B6 mice show that Ly6Chi monocytes are the major infected cell at the ear infection site from 1 day to 1 week postinfection, remaining infected thereafter, whereas DCs/macrophages predominate as infected cells upon 2 weeks of infection [41]. Recent results also suggest that both monocytes and monocyte-derived dendritic cells (Mo-DCs) may either express effector activity against Leishmania parasites or contribute to infection in different models. For example, Mo-DCs became infected at the infection site and migrate to LN to induce Th1 responses [32]. By contrast, DCs that were infected through uptake of infected neutrophils fail as APCs for CD4 T cells [41].
Nonetheless, the effector functions of both monocytes and DCs were recently evidenced in the L. major model (Table 1). Following L. major infection and platelet activation, Gr1+ monocytes from B6 mice are recruited to infection site through the CCR2 receptor [31]. Phagocytosis of L. major by monocytes ensues, followed by killing/disappearance of L. major parasites both in vitro and in vivo [31]. Although other mechanisms were not addressed, monocytes eliminated L. major infection in a Phox-dependent manner in vitro [31]. Ly6Chi monocytes elicited by L. major infection can also kill parasites in vitro by producing NO [42]. A possible explanation to reconcile these apparently discrepant results is that peroxynitrites derived from both NO and ROS are involved as a more effective killing mechanism [43]. Injection of purified monocytes at the time of infection also helped infected B6 mice to control skin lesions and L. major parasites [42]. Treatment with all-trans-retinoic acid (ATRA) to induce monocyte maturation into macrophages releases T cell proliferation from suppression mediated by NO-producing immature monocytes [42]. However, treatment with ATRA also reduces parasite killing in vitro and promotes infection in vivo [42]. These results indicate that monocytes from B6 mice are authentic effector cells against L. major infection and that failure to recruit monocytes in CCR2 deficient mice has a major contribution to susceptibility to infection [31, 42]. Resistance to L. major infection has also been attributed to monocyte derived INOS-producing inflammatory DCs [30, 39]. However, activated monocytes share many markers with inflammatory DCs, including CD11c [44], and current markers fail to achieve a clear distinction between these phenotypes in the infection site [32]. Although CD11c has been considered as a key marker for DCs, a recent investigation on depletion of cells in a CD11c-DTR model indicates that monocytes are also depleted and that conclusions drawn from these models should be interpreted with caution [44]. In any case, DCs predominate in lymph nodes where they help immunity both as NO-producing effector cells [30, 39] and APCs to induce Th1 responses in L. major infection [32].
Table 1.
The role of phagocytes and IL-4 in immunity to L. major infection.
| Phagocytes | Role in immunity | Experimental model | Outcome to infection | Ref. |
|---|---|---|---|---|
| Monocytes | APCs | Injected in B6 mice 4 wks upon infection | Generation of Mo-DCs/APCs (Th1 responses) | [32] |
| Monocytes | NO-producing effector cells | Injected in B6 mice upon infection | Resistance to acute infection | [42] |
| Monocytes | ROS-producing effector cells | B6-CCR2.KO | Susceptibility | [31] |
| DCs | APCs | B6-CCR2.KO | Susceptibility/Th2 response | [29] |
| DCs | APCs/NO-producing effector cells | B6-CCR2.KO | Defective recruitment of DCs to LN | [30] |
| DCs | APCs/NO-producing effector cells | CD11ccreIL-4Rα flox/- | Susceptibility/Th2 response | [39] |
| Macrophages | NO-producing effector cells | LysMcreIL-4Rα flox/- | Resistance to acute infection | [9] |
8. Protective Effects of IL-4 in L. major Infection
New evidence has challenged the role of IL-4 as the canonical Th2 cytokine that favors L. major infection. Early injection of IL-4 in susceptible mice promotes Th1 responses and resistance to L. major infection, through stimulation of DCs to produce IL-12 [45]. Similarly, in concert with TLR ligands, IL-4 and IL-4Rα induce priming of Th1 responses by DCs [46]. Nonetheless, IL-4 also alternatively activates DCs in Th2 responses [46], in analogy to IL-4-alternatively activated macrophages [9]. Therefore, the role of IL-4 is target cell and context dependent. Interestingly, BALB/c mice deficient for IL-4Rα expression on CD11c+ cells are highly susceptible to L. major infection [39]. These studies suggest that IL-4/IL-4Rα signaling accounts for resistance in the acute phase of infection, by promoting DC-induced Th1 responses and classical macrophage activation [39]. As discussed here, however, IL-4Rα deficiency in CD11c+ activated monocytes could also account for susceptibility to L. major infection. By contrast, defective IL-4Rα expression in macrophages results in increased resistance to L. major by blocking alternative activation of macrophages [9].
Recent studies demonstrate that exacerbated Th2 responses help resistance to L. major in Th1-biased B6 model and that late treatment with anti-IL-4 (2–4 wks) increases susceptibility to infection [47]. Interestingly, NO-producing Gr1+ monocytes accumulate in the spleens of L. major-infected mice in this mixed Th1/Th2 model (unpublished results). Moreover, similar to findings with macrophages [9, 20, 48], monocytes elicited by L. major can be stimulated with a combination of IL-4 and IFN-γ to kill parasites in a NO-dependent manner [42]. IL-4 and IL-13, which share the same IL-4Rα, induce myelomonopoiesis and therefore play a role both in the generation [49, 50] and the activation [51] of monocytes under certain conditions.
9. Concluding Remarks
Different phagocyte populations can be infected by L. major and express distinct responses that affect immunoregulation (Figure 2). Taken together, the studies discussed here also open new questions to the controversial role of IL-4/IL-4Rα in L. major model [52]. Furthermore, monocytes and DCs can be relocated to the core of this issue, with direct implications for the development of new vaccines to Leishmaniasis [53].
Figure 2.
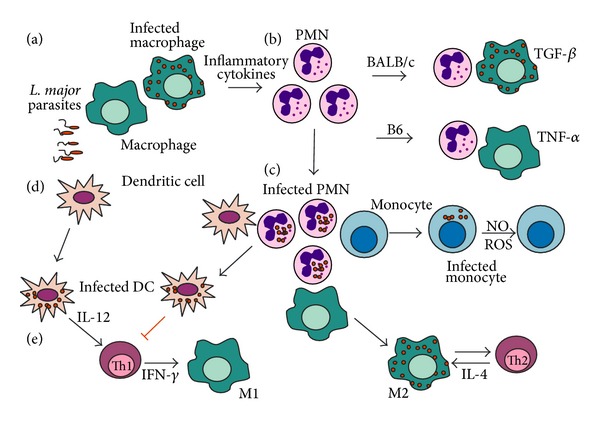
(a) Interactions among phagocytes in L. major infection. Upon L. major infection, tissue resident macrophages produce inflammatory cytokines and chemokines [4, 18] that recruit neutrophils and monocytes, which act as effector cells [31, 42] or give rise to inflammatory macrophages and DCs [30, 32]. (b) Neutrophils may either help or prevent parasite clearance by macrophages from B6 and BALB/c mice, respectively [3, 5]. (c) In addition, infected neutrophils help to propagate infection to macrophages, monocytes, and DCs [14, 41]. Moreover, macrophages primed by apoptotic neutrophils became permissive to subsequent infection and induce Th2 responses [21]. (d) DCs infected through efferocytosis of neutrophils fail to activate T lymphocytes [41]. Otherwise, infected DCs go to lymph nodes and induce Th1 responses [29, 32]. (e) Th1 cytokines activate M1 macrophages to kill parasites, whereas Th2 responses induce parasite-permissive M2 macrophages [6, 7].
Acknowledgments
The authors' cited work was financed by the Brazilian National Research Council (CNPq), the Rio de Janeiro State Science Foundation (FAPERJ), and the National Institutes of Science and Technology Initiative (INCT).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Matte C, Olivier M. Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. Journal of Infectious Diseases. 2002;185(5):673–681. doi: 10.1086/339260. [DOI] [PubMed] [Google Scholar]
- 2.Rabhi I, Rabhi S, Ben-Othman R, et al. Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view. PLoS Neglected Tropical Diseases. 2012;6(8) doi: 10.1371/journal.pntd.0001763.e1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro-Gomes FL, Otero AC, Gomes NA, et al. Macrophage interactions with neutrophils regulate Leishmania major infection. Journal of Immunology. 2004;172(7):4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro-Gomes FL, Moniz-De-Souza MCA, Borges VM, et al. Turnover of neutrophils mediated by Fas ligand drives Leishmania major infection. Journal of Infectious Diseases. 2005;192(6):1127–1134. doi: 10.1086/432764. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro-Gomes FL, Moniz-de-Souza MCA, Alexandre-Moreira MS, et al. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. Journal of Immunology. 2007;179(6):3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- 6.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual Review of Immunology. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 7.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hölscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. The Journal of Immunology. 2006;176(2):1115–1121. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 10.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in Leishmaniasis. Journal of Immunology. 2001;166(2):1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 11.Miles SA, Conrad SM, Alves RG, Jeronimo SMB, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania . Journal of Experimental Medicine. 2005;201(5):747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filardy AA, Pires DR, Dosreis GA. Macrophages and neutrophils cooperate in immune responses to Leishmania infection. Cellular and Molecular Life Sciences. 2011;68(11):1863–1870. doi: 10.1007/s00018-011-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beil WJ, Meinardus-Hager G, Neugebauer D-C, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. Journal of Leukocyte Biology. 1992;52(2):135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 14.Peters NC, Egen JG, Secundino N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. Journal of Immunology. 2001;167(9):5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 16.Wheat WH, Pauken KE, Morris RV, Titus RG. Lutzomyia longipalpis salivary peptide maxadilan alters murine dendritic cell expression of CD80/86, CCR7, and cytokine secretion and reprograms dendritic cell-mediated cytokine release from cultures containing allogeneic T cells. Journal of Immunology. 2008;180(12):8286–8298. doi: 10.4049/jimmunol.180.12.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohlbaum AM, Gregory MS, Ju ST, Marshak-Rothstein A. Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors. Journal of Immunology. 2001;167(11):6217–6224. doi: 10.4049/jimmunol.167.11.6217. [DOI] [PubMed] [Google Scholar]
- 18.Filardy AA, Costa-da-Silva AC, Koeller CM, et al. Infection with Leishmania major induces a cellular stress response in macrophages. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0085715.e85715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayagaki N, Yamaguchi N, Nagao F, et al. Polymorphism of murine Fas ligand that affects the biological activity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):3914–3919. doi: 10.1073/pnas.94.8.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogdan C, Stenger S, Rollinghoff M, Solbach W. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin 4 synergizes with interferon-γ to activate murine macrophages for killing of Leishmania majoramastigotes . European Journal of Immunology. 1991;21(2):327–333. doi: 10.1002/eji.1830210213. [DOI] [PubMed] [Google Scholar]
- 21.Filardy AA, Pires DR, Nunes MP, et al. Proinflammatory clearance of apoptotic neutrophils induces an IL-12lowIL-10high regulatory phenotype in macrophages. Journal of Immunology. 2010;185(4):2044–2050. doi: 10.4049/jimmunol.1000017. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Research. 2013;23(7):898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nature Reviews Immunology. 2002;2(12):965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 24.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. The Journal of Immunology. 2004;173(10):6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 25.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T H17 cell differentiation. Nature. 2009;458(7234):78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 26.Devaney JM, Greene CM, Taggart CC, Carroll TP, O'Neill SJ, McElvaney NG. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Letters. 2003;544(1–3):129–132. doi: 10.1016/s0014-5793(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 27.Benabid R, Wartelle J, Malleret L, et al. Neutrophil elastase modulates cytokine expression: contribution to host defense against pseudomonas aeruginosa induced pneumonia. The Journal of Biological Chemistry. 2012;287(42):34883–34894. doi: 10.1074/jbc.M112.361352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunology. 2006;7(3):311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 29.Sato N, Ahuja SK, Quinones M, et al. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells: absence of CCR2 shifts the Leishmania major—resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. Journal of Experimental Medicine. 2000;192(2):205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Trez C, Magez S, Akira S, Ryffel B, Carlier Y, Muraille E. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS Pathogens. 2009;5(6) doi: 10.1371/journal.ppat.1000494.e1000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goncalves R, Zhang X, Cohen H, Debrabant A, Mosser DM. Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. Journal of Experimental Medicine. 2011;208(6):1253–1265. doi: 10.1084/jem.20101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.León B, López-Bravo M, Ardavín C. Monocyte -derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania . Immunity. 2007;26(4):519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Mirkovich AM, Galelli A, Allison AC, Modabber FZ. Increased myelopoiesis during Leishmania major infection in mice: generation of ”safe targets“, a possible way to evade the immune mechanism. Clinical & Experimental Immunology. 1986;64(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Sunderkötter C, Nikolic T, Dillon MJ, et al. Subpopulations of Mouse Blood Monocytes Differ in Maturation Stage and Inflammatory Response. Journal of Immunology. 2004;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 35.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 36.Sunderkotter C, Kunz M, Steinbrink K, et al. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. The Journal of Immunology. 1993;151(9):4891–4901. [PubMed] [Google Scholar]
- 37.Steinbrink K, Schönlau F, Rescher U, et al. Ineffective elimination of Leishmania major by inflammatory (MRP14-positive) subtype of monocytic cells. Immunobiology. 2000;202(5):442–459. doi: 10.1016/s0171-2985(00)80103-5. [DOI] [PubMed] [Google Scholar]
- 38.Goto Y, Sanjoba C, Arakaki N, et al. Accumulation of macrophages expressing MRP8 and MRP14 in skin lesions during Leishmania major infection in BALB/c and RAG-2 knockout mice. Parasitology International. 2007;56(3):231–234. doi: 10.1016/j.parint.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Hurdayal R, Nieuwenhuizen NE, Revaz-Breton M, et al. Deletion of IL-4 receptor alpha on dendritic cells renders BALB/c mice hypersusceptible to Leishmania major infection. PLOS Pathogens. 2013;9(10) doi: 10.1371/journal.ppat.1003699.e1003699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro-Gomes FL, Roma EH, Carneiro MB, Doria NA, Sacks DL, Peters NC. Site dependent recruitment of inflammatory cells determines the effective dose of Leishmania major . Infection and Immunity. 2014;82(7):2713–2727. doi: 10.1128/IAI.01600-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro-Gomes FL, Peters NC, Debrabant A, Sacks DL. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathogens. 2012;8(2) doi: 10.1371/journal.ppat.1002536.e1002536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira WF, Ribeiro-Gomes FL, Guillermo LVC, et al. Myeloid-derived suppressor cells help protective immunity to Leishmania major infection despite suppressed T cell responses. Journal of Leukocyte Biology. 2011;90(6):1191–1197. doi: 10.1189/jlb.1110608. [DOI] [PubMed] [Google Scholar]
- 43.Linares E, Giorgio S, Mortara RA, Santos CXC, Yamada AT, Augusto O. Role of peroxynitrite in macrophage microbicidal mechanisms in vivo revealed by protein nitration and hydroxylation. Free Radical Biology and Medicine. 2001;30(11):1234–1242. doi: 10.1016/s0891-5849(01)00516-0. [DOI] [PubMed] [Google Scholar]
- 44.Meredith MM, Liu K, Darrasse-Jeze G, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. Journal of Experimental Medicine. 2012;209(6):1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biedermann T, Zimmermann S, Himmelrich H, et al. IL-4 instructs THI responses and resistance to Leishmania major in susceptible BALB/c mice. Nature Immunology. 2001;2(11):1054–1060. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 46.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):9977–9982. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira-Manfro WF, Ribeiro-Gomes FL, Filardy AA, et al. Inhibition of caspase-8 activity promotes protective Th1- and Th2-mediated immunity to Leishmania major infection. Journal of Leukococyte Biology. 2014;95(2):347–355. doi: 10.1189/jlb.0912463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenger S, Solbach W, Rollinhoff M, Bogdan C. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-α production by macrophages is induced by the synergistic action of interferon (IFN)-γ and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-γ and IL 4. European Journal of Immunology. 1991;21(7):1669–1675. doi: 10.1002/eji.1830210713. [DOI] [PubMed] [Google Scholar]
- 49.Lai YH, Heslan J, Poppema S, Elliott JF, Mosmann TR. Continuous administration of IL-13 to mice induces extramedullary hemopoiesis and monocytosis. Journal of Immunology. 1996;156(9):3166–3173. [PubMed] [Google Scholar]
- 50.Jacobsen SEW, Okkenhaug C, Veiby OP, Caput D, Ferrara P, Minty A. Interleukin 13: novel role in direct regulation of proliferation and differentiation of primitive hematopoietic progenitor cells. Journal of Experimental Medicine. 1994;180(1):75–82. doi: 10.1084/jem.180.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. Journal of Clinical Investigation. 2006;116(10):2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurdayal R, Brombacher F. The role of IL-4 and IL-13 in cutaneous Leishmaniasis. Immunology Letters. 2014;161(1) doi: 10.1016/j.imlet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 53.Masic A, Hurdayal R, Nieuwenhuizen NE, Brombacher F, Moll H. Dendritic cell-mediated vaccination relies on interleukin-4 receptor signaling to avoid tissue damage after Leishmania major infection of BALB/c mice. PLoS Neglected Tropical Diseases. 2012;6(7) doi: 10.1371/journal.pntd.0001721.e1721 [DOI] [PMC free article] [PubMed] [Google Scholar]


