Table 2. Substrate Scope of the Asymmetric Fluorination of 2-Aryl Cyclohexanonesa,b,c.
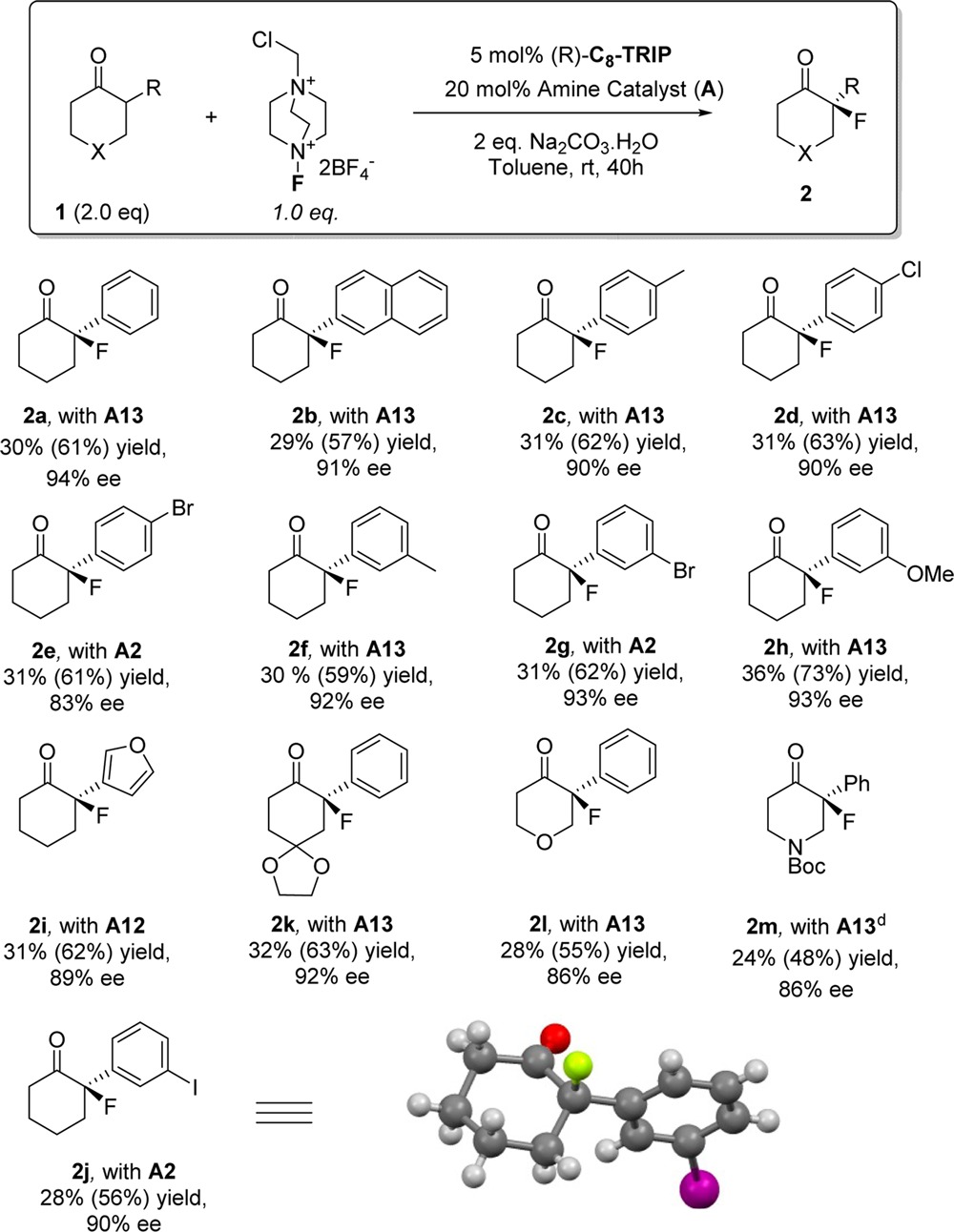
Reactions were carried out with ketone (0.2 mmol), Selectfluor (0.1 mmol) amine catalyst (0.02 mmol), phosphoric acid catalyst (0.005 mmol), and Na2CO3·H2O (0.2 mmol) in toluene (1.0 mL) for 40 h at rt.
Yields are isolated yields after chromatography. Primary yields calculated based on the excess ketone employed. Yields in parentheses calculated based on Selectfluor, the limiting reagent in all cases.
Absolute configurations assigned by analogy to 2j, determined by X-ray crystallography.
Characterized as the N-tosyl derivative.
