Table 2.
Screening various ligands and the optimization of reaction conditions.
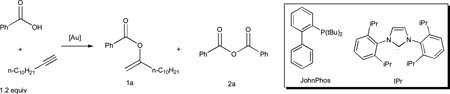 | ||||||
|---|---|---|---|---|---|---|
| entry | [Au] | Solvent, Temperature, Conc. |
time | NMR yielda | ||
| 1a | TON | 2a | ||||
| 1 | IPrAuNTf2 (1%) | DCE, 40 °C, 0.5 M | 8 h | 0.5% | 0.5 | 0 |
| 2 | L1AuCl/AgNTf2 (1.1%/1%) | DCE, 40 °C, 0.5 M | 8 h | 6% | 5 | 0 |
| 3 | L2AuCl/AgNTf2 (1.1%/1%) | DCE, 40 °C, 0.5 M | 8 h | trace | - | 0 |
| 4 | L3AuCl/AgNTf2 (1.1%/1%) | DCE, 40 °C, 0.5 M | 8 h | 89% | 80 | 7% |
| 5 | L7AuCl/AgNTf2 (1.1%/1%) | DCE, 40 °C, 0.5 M | 8 h | 89% | 80 | 7% |
| 6 | L3AuCl/AgNTf2 (0.11%/0.1%) | DCE, 40 °C, 0.5 M | 8 h | 67% | 609 | 0 |
| 7 | L7AuCl/ AgNTf2 (0.11%/0.1%) | DCE, 40 °C, 0.5 M | 8 h | 91% | 827 | 2% |
| 8 | L7AuCl/AgNTf2 (220/200 ppm) | DCE, 40 °C, 1 M | 12 h | 37% | 1681 | 0 |
| 9 | L4AuCl/AgNTf2 (220/200 ppm) | DCE, 40 °C, 1 M | 12 h | 27% | 1227 | 0 |
| 10 | L5AuCl/AgNTf2 (220/200 ppm) | DCE, 40 °C, 1 M | 12 h | 35% | 1590 | 0 |
| 11 | L6AuCl/AgNTf2 (220/200 ppm) | DCE, 40 °C, 1 M | 12 h | 36% | 1636 | 0 |
| 12 | L8AuCl/AgNTf2 (220/200 ppm) | DCE, 40 °C, 1 M | 12 h | 43% | 1954 | 0 |
| 13 | L8AuNTf2 (40 ppm)/ NaBArF (0.12 %) | PhF, 80 °C, 2 M | 12 h | 97% | 24250 | 0 |
| 14 | L8AuNTf2 (25 ppm)/ NaBArF (0.12 %) | PhF, 80 °C, 2 M | 12 h | 86% | 34400 | 0 |
| 15 | JohnPhosAuNTf2 (500 ppm)/ NaBArF (0.12 %) | PhF, 80 °C, 2 M | 12 h | 2% | 40 | 0 |
The NMR yield calculated by assuming that the triplet at around 0.9 ppm corresponds to the terminal methyl groups of all compounds derived from 2-dodecyne. NaBARF, sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate; DCE, 1,2-dichloroethane.
