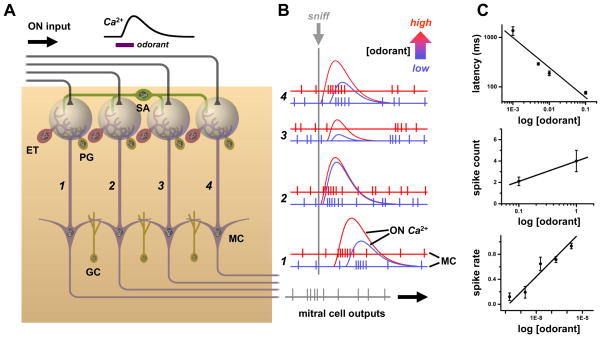
Figure 2. Spatial and temporal coding of odorant concentration in the olfactory bulb.
A. Schematic of four parallel glomerular odor-encoding channels. During responses to odorant stimuli sampled by a single sniff, olfactory nerve (ON) spike inputs to different glomeruli exhibit different latencies and time courses (monitored by calcium indicator fluorescence signals). This reflects the diversity of olfactory receptor molecular tunings. These spatiotemporal patterns of input are processed by glomerular neural circuits, including excitatory external tufted (ET), as well as inhibitory periglomerular (PG) and short axon (SA) cells, before being encoded into spiking patterns of mitral cells (MC) for relay to olfactory cortex. The SA cells have widespread interglomerular projections that could act to normalize spatial patterns of glomerular output as input patterns vary with odorant concentration (c.f. Fig. 5B). A second layer of signal filtering and pattern transformation occurs when granule cells (GC) or other local interneurons inhibit mitral cells. B. As odorant concentration is increased from low (blue) to high (red), OSNs are activated more rapidly and presynaptic glomerular responses have shorter latencies (c.f. red ON traces). Mitral cell spike output may track these latency shifts over the sniff cycle (c.f. left-shifted red spike responses in glomerular channels 1, 3 & 4). Such phase shifts of spike latency over sniff cycles may encode odor concentration, while leaving invariant overall patterns of relative latencies between glomeruli (which can encode odor quality, as latencies will vary with different sensitivities of receptors to different odorants). This simple scheme allows early separation of the coding of odor concentration vs. odor quality, but may be complicated by lateral inhibition between glomeruli. Some mitral cells exhibit suppressed basal spiking in response to an odor, which may be followed by rebound spiking whose latency becomes longer as odorant concentration is increased (due to stronger inhibition) (as pictured in glomerular channel 2). C. Example of excitatory mitral cell response in a glomerular coding channel. Data show a logarithmic decrement of mitral cell spike latency with increasing concentration, which provides an immediate readout of intensity (upper panel). Mitral cell spike count and mean spike rate over a sniff (middle and lower panels) increase, so spike rate coding of concentration is possible if readouts have longer integration times. Data sources: C: upper panel: [116], salamander mitral/ tufted cell excitatory response to isoamyl acetate; middle panel: [41], mean spikes per sniff cycle for rat mitral/ tufted cell response to cineole; lower panel: [117], mouse mitral/ tufted cell spike response evoked by heptanal activation of olfactory receptor I7 (mean of 8 cells, spike rate increment over baseline rate, normalized to maximum rate).