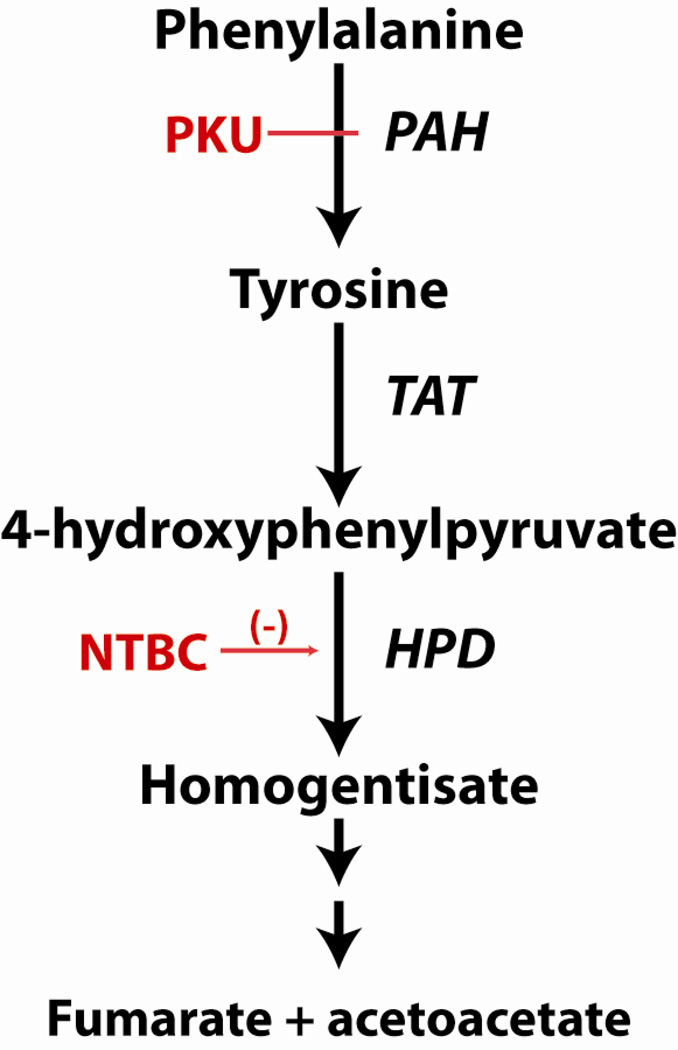
Figure 1. Phenylalanine Hydroxylation and the Tyrosine Degradation Pathway.
L-Phenylalanine is hydroxylated to L-tyrosine, primarily in liver, via the activity of phenylalanine hydroxylase (PAH, EC 1.14.16.1). Phenylketonuria (PKU) is caused by recessively inherited mutations in the Pah gene associated with reduced or absent PAH activity. Hyperphenylalaninemia is the result. L-Tyrosine is degraded to fumarate and acetoacetate beginning with deamination through the activity of tyrosine aminotransferase (TAT, EC 2.6.1.5). 4-hydroxyphenylpyruvate dioxygenase (HPD, EC 1.13.11.27) catalyzes the next step in L-tyrosine degradation, namely the oxidation of 4-hydroxyphenylpyruvate to homogentisate. 2-[2-nitro-4- (trifluoromethyl)benzoyl]cyclohexane-1,3-dione (NTBC, also known as nitisinone) is a reversible inhibitor of HPD activity. Oral administration of NTBC blocks the production of homogentisate and downstream metabolites; NTBC is therefore used in the treatment of the human inborn errors of metabolism alkaptonuria and tyrosinemia type 1. Blood tyrosine concentration is increased in mice and humans treated with NTBC.