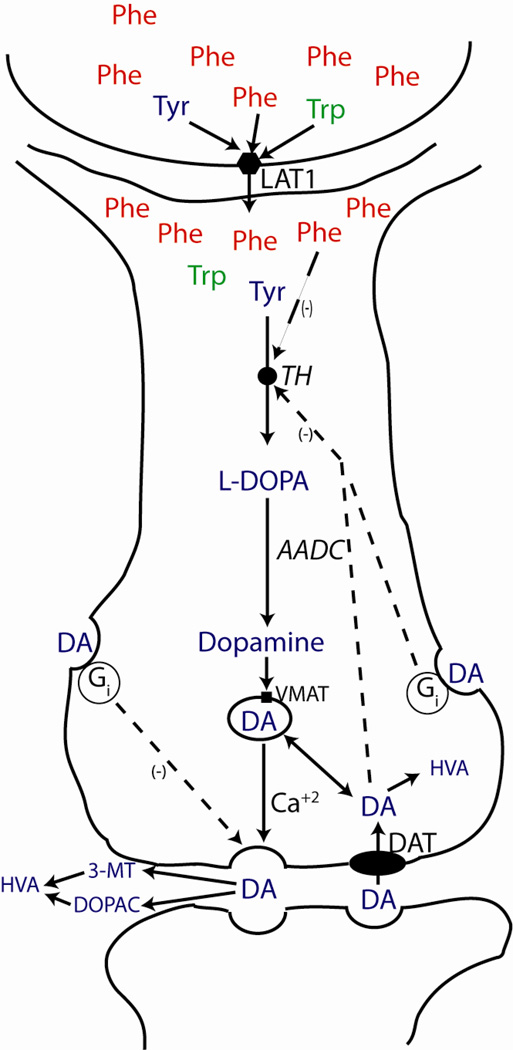
Figure 2. Monoamine neurotransmitter synthesis and regulation in neurons.
L-tyrosine ( ) and L-tryptophan (
) and L-tryptophan ( ) are the substrates for dopamine (
) are the substrates for dopamine ( ) and serotonin synthesis respectively. Both cross the blood brain barrier and enter neurons via diffusion facilitated by the LAT1 neutral amino acid transporter in competition with L-phenylalanine (
) and serotonin synthesis respectively. Both cross the blood brain barrier and enter neurons via diffusion facilitated by the LAT1 neutral amino acid transporter in competition with L-phenylalanine ( ) and other large neutral amino acids. Tyr is converted to L-DOPA by tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis. TH activity is stimulated by Tyr, but inhibited through Phe-mediated competition, DA or L-DOPA-mediated feedback inhibition, and via a DA-activated, G-protein coupled, synthesis-modulating autoreceptor. TH activity is also regulated through reversible phosphorylation triggered by a variety of stimuli (not shown). DA is synthesized from L-DOPA by aromatic amino acid decarboxylase (AADC), taken up into secretory vesicles via a monoamine specific transporter (VMAT) and secreted into the synapse following Ca+2 influx in response to a nerve stimulus. Neurotransmission terminates through DA reuptake via a specific transporter (DAT) or through degradation of dopamine to homovanillic acid (HVA). Extracellular DA can also act via another G protein-coupled autoreceptor to suppress further synaptic dopamine release. The production of HVA from DA requires both oxidation via the activity of monoamine oxidase (MAO) and methylation by catechol-O-methyl-transferase (COMT), but these sequential reactions can occur in either order. DA oxidation occurring first produces 3,4- dihydroxyphenylacetic acid (DOPAC) followed by methylation to HVA; initial DA methylation yields 3-methoxytyramine (3-MT) that is subsequently oxidized to HVA. The brain content of DOPAC and 3-MT are indicators of neuronal and synaptic DA turnover. Analogous mechanisms regulate the synthesis and secretion of serotonin from Trp followed by degradation to 5-hydroxyindoleacetic acid (5-HIAA) (not shown).
) and other large neutral amino acids. Tyr is converted to L-DOPA by tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis. TH activity is stimulated by Tyr, but inhibited through Phe-mediated competition, DA or L-DOPA-mediated feedback inhibition, and via a DA-activated, G-protein coupled, synthesis-modulating autoreceptor. TH activity is also regulated through reversible phosphorylation triggered by a variety of stimuli (not shown). DA is synthesized from L-DOPA by aromatic amino acid decarboxylase (AADC), taken up into secretory vesicles via a monoamine specific transporter (VMAT) and secreted into the synapse following Ca+2 influx in response to a nerve stimulus. Neurotransmission terminates through DA reuptake via a specific transporter (DAT) or through degradation of dopamine to homovanillic acid (HVA). Extracellular DA can also act via another G protein-coupled autoreceptor to suppress further synaptic dopamine release. The production of HVA from DA requires both oxidation via the activity of monoamine oxidase (MAO) and methylation by catechol-O-methyl-transferase (COMT), but these sequential reactions can occur in either order. DA oxidation occurring first produces 3,4- dihydroxyphenylacetic acid (DOPAC) followed by methylation to HVA; initial DA methylation yields 3-methoxytyramine (3-MT) that is subsequently oxidized to HVA. The brain content of DOPAC and 3-MT are indicators of neuronal and synaptic DA turnover. Analogous mechanisms regulate the synthesis and secretion of serotonin from Trp followed by degradation to 5-hydroxyindoleacetic acid (5-HIAA) (not shown).