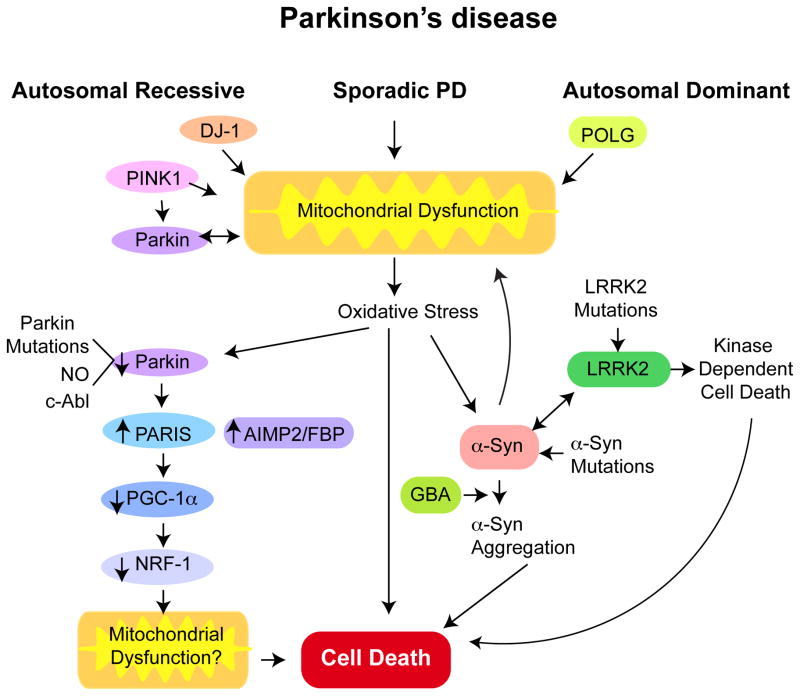
Figure 2. Potential pathogenic pathways linking the genetic and sporadic causes of PD.
A key feature of both sporadic and genetic causes of PD is mitochondrial dysfunction. Mutations in the autosomal recessive genes PINK1, Parkin and DJ-1 may directly cause mitochondrial dysfunction. PINK1 may act upstream of Parkin. PINK1 and Parkin may regulate mitochondria mitophagy. Parkin may directly regulate mitochondrial biogenesis through PARIS, which is a transcriptional repressor of the PGC-1α, the master regulator of mitochondrial biogenesis. Dominant mutations in POLG, the catalytic subunit of mitochondrial DNA polymerase causes Parkinsonism in some families. Dominant mutations in LRRK2 and α-synuclein (α-syn) cause PD. Oxidative stress including nitrosative stress and c-Abl phosphorylation of Parkin leads to its inactivation in sporadic PD and subsequent accumulation of substrates that are degraded by the ubiquitin proteasome system, such as AIMP2, FBP1 and PARIS. PARIS may be the key pathogenic Parkin substrate as knockdown of PARIS in an adult conditional knockout of Parkin completely rescues neurodegeneration of DA neurons. α-Synuclein aggregation is a key step in DA neuron degeneration in PD. Oxidative and nitrosative (NO) stress can accelerate α-synuclein and aggregated α-synuclein can damage mitochondria setting in motion a feed forward mechanism. GBA mutations also seem to accelerate α-synuclein aggregation and LRRK2 and α-synuclein interact at some level in the pathogenesis of PD. LRRK2 mutations lead to DA neurodegeneration that is kinase dependent.