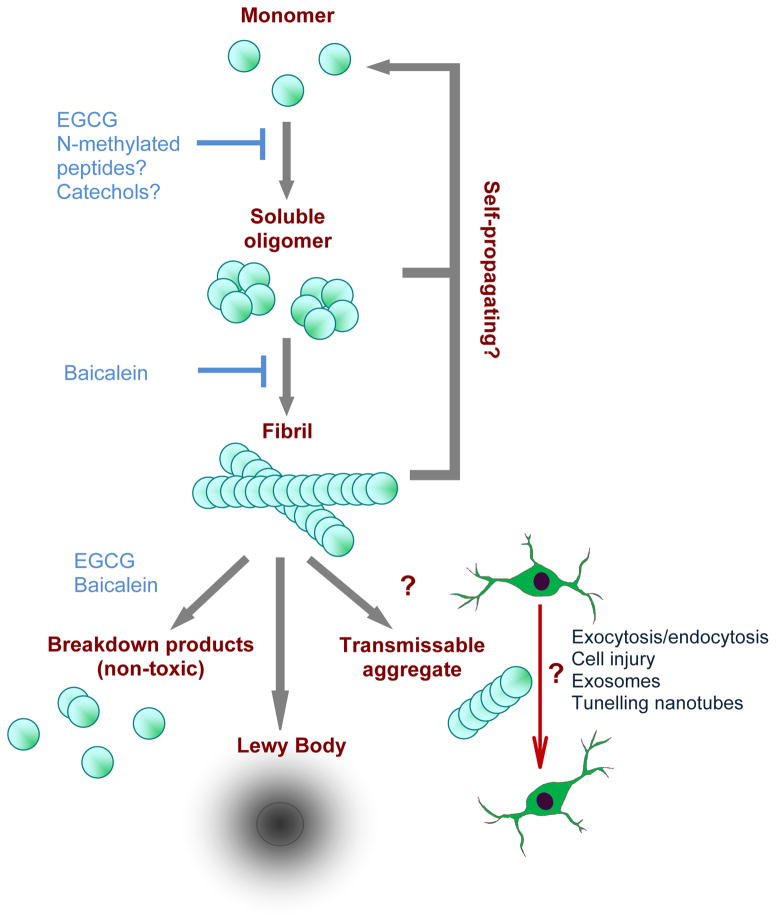
Figure 3. α-Synuclein aggregation and pathophysiology.
α-Synuclein natively unfolded monomers assemble to form β-sheet-rich soluble oligomers, which further aggregate to form mature fibrils. Aggregates at both stages are thought to act as templates that seed further α-synuclein fibrillogenesis in a feed-forward cycle. Fibrils can deposit into Lewy bodies or might break down into small transmissible aggregates via incomplete degradation that are able to transmit between cells, facilitating the spread of α-synuclein aggregation and toxicity in a prion-like manner. The mechanism of transmission from one neuron to another is unknown. Prevention of oligomer and fibril formation and the disaggregation of mature fibrils to non-toxic breakdown products are therapeutic goals and several compounds, including EGCG, baicalein, N-methylated peptides and catechol-based compounds have been put forward based on their effectiveness in vitro.