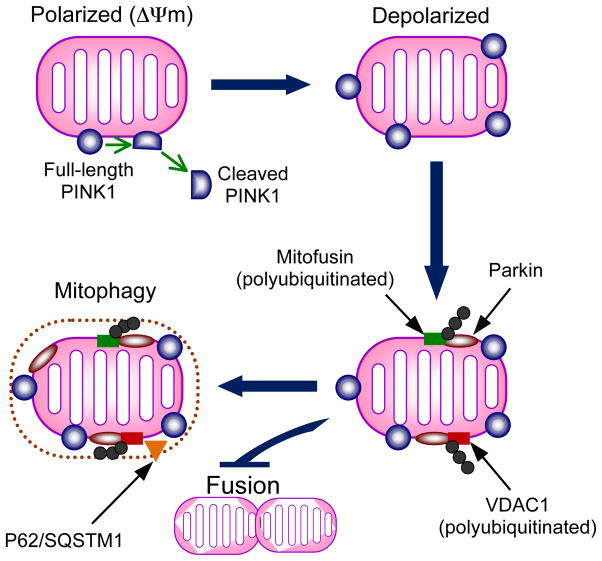
Figure 5. Interaction of PINK1 and Parkin in regulating mitochondrial turnover.
The model proposed is based on recent evidence supporting PINK1-dependent recruitment of Parkin to mitochondria and subsequent Parkin substrate polyubiquitination to promote autophagy. On polarized mitochondria (mitochondrial membrane potential intact), PINK1 is cleaved into a short ~52 kDa fragment, which is released into the cytosol. This cleavage is voltage-dependent and impaired by membrane depolarization in dysfunctional mitochondria. Retention of PINK1 at the mitochondrial membrane leads to recruitment of Parkin by unknown mechanisms. Once localized to mitochondria, Parkin polyubiquitylates mitofusin and VDAC1 (voltage-dependent anion channel) and VDAC1 is required for Parkin-mediated mitochondrial clearance. Polyubiqutination of mitofusin may inactivate it and prevent fusion of the dysfunctional mitochondrion with the pool of healthy mitochondria. The autophagic adaptor protein P62/SQSTM1 is recruited to mitochondria and is required for mitochondrial clearance.